Integrative approach to sporadic Alzheimer's disease: deficiency of TYROBP in a tauopathy mouse model reduces C1q and normalizes clinical phenotype while increasing spread and state of phosphorylation of tau
- PMID: 30283031
- PMCID: PMC6447470
- DOI: 10.1038/s41380-018-0258-3
Integrative approach to sporadic Alzheimer's disease: deficiency of TYROBP in a tauopathy mouse model reduces C1q and normalizes clinical phenotype while increasing spread and state of phosphorylation of tau
Abstract
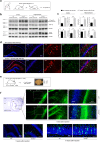
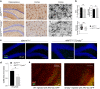
TYROBP/DAP12 forms complexes with ectodomains of immune receptors (TREM2, SIRPβ1, CR3) associated with Alzheimer's disease (AD) and is a network hub and driver in the complement subnetwork identified by multi-scale gene network studies of postmortem human AD brain. Using transgenic or viral approaches, we characterized in mice the effects of TYROBP deficiency on the phenotypic and pathological evolution of tauopathy. Biomarkers usually associated with worsening clinical phenotype (i.e., hyperphosphorylation and increased tauopathy spreading) were unexpectedly increased in MAPTP301S;Tyrobp-/- mice despite the improved learning behavior and synaptic function relative to controls with normal levels of TYROBP. Notably, levels of complement cascade initiator C1q were reduced in MAPTP301S;Tyrobp-/- mice, consistent with the prediction that C1q reduction exerts a neuroprotective effect. These observations suggest a model wherein TYROBP-KO-(knock-out)-associated reduction in C1q is associated with normalized learning behavior and electrophysiological properties in tauopathy model mice despite a paradoxical evolution of biomarker signatures usually associated with neurological decline.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



References
-
- Readhead B, Haure-Mirande JV, Zhang B, Haroutunian V, Gandy S, Schadt E, et al. Molecular systems evaluation of oligomerogenic APPE693Q and fibrillogenic APPKM670/671NL/PSEN1Δexon9 mouse models identifies shared features with human Alzheimer’s brain molecular pathology. Mol Psychiatry. 2015;21:1099–111. doi: 10.1038/mp.2015.167. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

