Adolescent exposure to Δ9-tetrahydrocannabinol alters the transcriptional trajectory and dendritic architecture of prefrontal pyramidal neurons
- PMID: 30283037
- PMCID: PMC6430678
- DOI: 10.1038/s41380-018-0243-x
Adolescent exposure to Δ9-tetrahydrocannabinol alters the transcriptional trajectory and dendritic architecture of prefrontal pyramidal neurons
Abstract
Neuronal circuits within the prefrontal cortex (PFC) mediate higher cognitive functions and emotional regulation that are disrupted in psychiatric disorders. The PFC undergoes significant maturation during adolescence, a period when cannabis use in humans has been linked to subsequent vulnerability to psychiatric disorders such as addiction and schizophrenia. Here, we investigated in a rat model the effects of adolescent exposure to Δ9-tetrahydrocannabinol (THC), a psychoactive component of cannabis, on the morphological architecture and transcriptional profile of layer III pyramidal neurons-using cell type- and layer-specific high-resolution microscopy, laser capture microdissection and next-generation RNA-sequencing. The results confirmed known normal expansions in basal dendritic arborization and dendritic spine pruning during the transition from late adolescence to early adulthood that were accompanied by differential expression of gene networks associated with neurodevelopment in control animals. In contrast, THC exposure disrupted the normal developmental process by inducing premature pruning of dendritic spines and allostatic atrophy of dendritic arborization in early adulthood. Surprisingly, there was minimal overlap of the developmental transcriptomes between THC- and vehicle-exposed rats. THC altered functional gene networks related to cell morphogenesis, dendritic development, and cytoskeleton organization. Marked developmental network disturbances were evident for epigenetic regulators with enhanced co-expression of chromatin- and dendrite-related genes in THC-treated animals. Dysregulated PFC co-expression networks common to both the THC-treated animals and patients with schizophrenia were enriched for cytoskeletal and neurite development. Overall, adolescent THC exposure altered the morphological and transcriptional trajectory of PFC pyramidal neurons, which could enhance vulnerability to psychiatric disorders.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
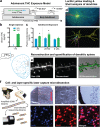
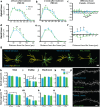
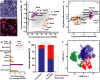


References
Publication types
MeSH terms
Substances
Grants and funding
- R01 AG050986/AG/NIA NIH HHS/United States
- R01-DA033660/U.S. Department of Health & Human Services | NIH | National Institute on Drug Abuse (NIDA)/International
- R01-AG050986/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)/International
- I01 BX002395/BX/BLRD VA/United States
- R01-DA030359/U.S. Department of Health & Human Services | NIH | National Institute on Drug Abuse (NIDA)/International
- R01 DA033660/DA/NIDA NIH HHS/United States
- T32 GM007280/GM/NIGMS NIH HHS/United States
- R01 DA030359/DA/NIDA NIH HHS/United States
- 20540/Brain and Behavior Research Foundation (Brain & Behavior Research Foundation)/International
- P01 DA047233/DA/NIDA NIH HHS/United States
- BX002395/U.S. Department of Veterans Affairs (VA)/International
- R01-MH109677/U.S. Department of Health & Human Services | NIH | National Institute of Mental Health (NIMH)/International
- NIRG-340998/ALZ/Alzheimer's Association/United States
- S10 OD018522/OD/NIH HHS/United States
- R01 MH109677/MH/NIMH NIH HHS/United States
- F30 DA038954/DA/NIDA NIH HHS/United States
- F30-DA038954/U.S. Department of Health & Human Services | NIH | National Institute on Drug Abuse (NIDA)/International
LinkOut - more resources
Full Text Sources
Miscellaneous

