Antibody and TLR7 agonist delay viral rebound in SHIV-infected monkeys
- PMID: 30283138
- PMCID: PMC6237629
- DOI: 10.1038/s41586-018-0600-6
Antibody and TLR7 agonist delay viral rebound in SHIV-infected monkeys
Erratum in
-
Publisher Correction: Antibody and TLR7 agonist delay viral rebound in SHIV-infected monkeys.Nature. 2018 Dec;564(7734):E8. doi: 10.1038/s41586-018-0721-y. Nature. 2018. PMID: 30397346
Abstract
The latent viral reservoir is the critical barrier for the development of a cure for HIV-1 infection. Previous studies have shown direct antiviral activity of potent HIV-1 Env-specific broadly neutralizing antibodies (bNAbs) administered when antiretroviral therapy (ART) was discontinued, but it remains unclear whether bNAbs can target the viral reservoir during ART. Here we show that administration of the V3 glycan-dependent bNAb PGT121 together with the Toll-like receptor 7 (TLR7) agonist vesatolimod (GS-9620) during ART delayed viral rebound following discontinuation of ART in simian-human immunodeficiency virus (SHIV)-SF162P3-infected rhesus monkeys in which ART was initiated during early acute infection. Moreover, in the subset of monkeys that were treated with both PGT121 and GS-9620 and that did not show viral rebound after discontinuation of ART, adoptive transfer studies and CD8-depletion studies also did not reveal virus. These data demonstrate the potential of bNAb administration together with innate immune stimulation as a possible strategy for targeting the viral reservoir.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

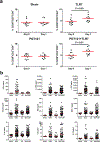


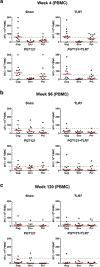


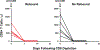








Comment in
-
Combination treatment prevents HIV re-emergence in monkeys.Nature. 2018 Nov;563(7731):333-335. doi: 10.1038/d41586-018-06818-y. Nature. 2018. PMID: 30425354 No abstract available.
References
-
- Finzi D et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science 278, 1295–1300 (1997). - PubMed
-
- Ho YC et al. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell 155, 540–551, 10.1016/j.cell.2013.09.020 (2013). - DOI - PMC - PubMed
-
- Finzi D et al. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med 5, 512–517, 10.1038/8394 (1999). - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

