SK2 Channels Associate With mGlu1α Receptors and CaV2.1 Channels in Purkinje Cells
- PMID: 30283304
- PMCID: PMC6156379
- DOI: 10.3389/fncel.2018.00311
SK2 Channels Associate With mGlu1α Receptors and CaV2.1 Channels in Purkinje Cells
Abstract
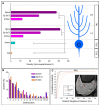
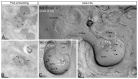
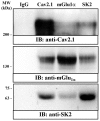
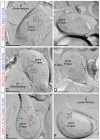
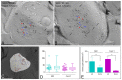
The small-conductance, Ca2+-activated K+ (SK) channel subtype SK2 regulates the spike rate and firing frequency, as well as Ca2+ transients in Purkinje cells (PCs). To understand the molecular basis by which SK2 channels mediate these functions, we analyzed the exact location and densities of SK2 channels along the neuronal surface of the mouse cerebellar PCs using SDS-digested freeze-fracture replica labeling (SDS-FRL) of high sensitivity combined with quantitative analyses. Immunogold particles for SK2 were observed on post- and pre-synaptic compartments showing both scattered and clustered distribution patterns. We found an axo-somato-dendritic gradient of the SK2 particle density increasing 12-fold from soma to dendritic spines. Using two different immunogold approaches, we also found that SK2 immunoparticles were frequently adjacent to, but never overlap with, the postsynaptic density of excitatory synapses in PC spines. Co-immunoprecipitation analysis demonstrated that SK2 channels form macromolecular complexes with two types of proteins that mobilize Ca2+: CaV2.1 channels and mGlu1α receptors in the cerebellum. Freeze-fracture replica double-labeling showed significant co-clustering of particles for SK2 with those for CaV2.1 channels and mGlu1α receptors. SK2 channels were also detected at presynaptic sites, mostly at the presynaptic active zone (AZ), where they are close to CaV2.1 channels, though they are not significantly co-clustered. These data demonstrate that SK2 channels located in different neuronal compartments can associate with distinct proteins mobilizing Ca2+, and suggest that the ultrastructural association of SK2 with CaV2.1 and mGlu1α provides the mechanism that ensures voltage (excitability) regulation by distinct intracellular Ca2+ transients in PCs.
Keywords: calcium channel; cerebellum; electron microscopy; immunohistochemistry; mGlu receptor; potassium channel; synapse.
Figures


Similar articles
-
Differential association of GABAB receptors with their effector ion channels in Purkinje cells.Brain Struct Funct. 2018 Apr;223(3):1565-1587. doi: 10.1007/s00429-017-1568-y. Epub 2017 Nov 25. Brain Struct Funct. 2018. PMID: 29177691 Free PMC article.
-
Quantitative localization of Cav2.1 (P/Q-type) voltage-dependent calcium channels in Purkinje cells: somatodendritic gradient and distinct somatic coclustering with calcium-activated potassium channels.J Neurosci. 2013 Feb 20;33(8):3668-78. doi: 10.1523/JNEUROSCI.2921-12.2013. J Neurosci. 2013. PMID: 23426693 Free PMC article.
-
Localization of SK2 channels relative to excitatory synaptic sites in the mouse developing Purkinje cells.Front Neuroanat. 2014 Dec 15;8:154. doi: 10.3389/fnana.2014.00154. eCollection 2014. Front Neuroanat. 2014. PMID: 25565979 Free PMC article.
-
SK2 channel expression and function in cerebellar Purkinje cells.J Physiol. 2011 Jul 15;589(Pt 14):3433-40. doi: 10.1113/jphysiol.2011.205823. Epub 2011 Apr 26. J Physiol. 2011. PMID: 21521760 Free PMC article. Review.
-
Presynaptic calcium channels.Neurosci Res. 2018 Feb;127:33-44. doi: 10.1016/j.neures.2017.09.012. Epub 2018 Jan 6. Neurosci Res. 2018. PMID: 29317246 Review.
Cited by
-
The Number and Distinct Clustering Patterns of Voltage-Gated Calcium Channels in Nerve Terminals.Front Neuroanat. 2022 Feb 24;16:846615. doi: 10.3389/fnana.2022.846615. eCollection 2022. Front Neuroanat. 2022. PMID: 35280978 Free PMC article.
-
Nanoscale Phosphoinositide Distribution on Cell Membranes of Mouse Cerebellar Neurons.J Neurosci. 2023 Jun 7;43(23):4197-4216. doi: 10.1523/JNEUROSCI.1514-22.2023. Epub 2023 May 9. J Neurosci. 2023. PMID: 37160366 Free PMC article.
-
Restoring endogenous Dlg4/PSD95 expression by an artificial transcription factor ameliorates cognitive and motor learning deficits in the R6/2 mouse model of Huntington's disease.Clin Epigenetics. 2025 Jun 12;17(1):100. doi: 10.1186/s13148-025-01903-2. Clin Epigenetics. 2025. PMID: 40506760 Free PMC article.
-
Gold In-and-Out: A Toolkit for Analyzing Subcellular Distribution of Immunogold-Labeled Membrane Proteins in Freeze-Fracture Replica Images.Front Neuroanat. 2022 Apr 4;16:855218. doi: 10.3389/fnana.2022.855218. eCollection 2022. Front Neuroanat. 2022. PMID: 35444519 Free PMC article.
-
Is Purkinje Neuron Hyperpolarisation Important for Cerebellar Synaptic Plasticity? A Retrospective and Prospective Analysis.Cerebellum. 2020 Dec;19(6):869-878. doi: 10.1007/s12311-020-01164-0. Cerebellum. 2020. PMID: 32654026 Review.
References
-
- Altman J., Bayer S. A. (1997). Development of the Cerebellar System in Relation to its Evolution, Structure and Functions. New York, NY: CRC Press.
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

