Palatable Food Dampens the Long-Term Behavioral and Endocrine Effects of Juvenile Stressor Exposure but May Also Provoke Metabolic Syndrome in Rats
- PMID: 30283308
- PMCID: PMC6156124
- DOI: 10.3389/fnbeh.2018.00216
Palatable Food Dampens the Long-Term Behavioral and Endocrine Effects of Juvenile Stressor Exposure but May Also Provoke Metabolic Syndrome in Rats
Abstract
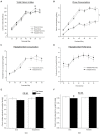
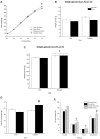
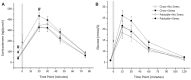
The juvenile period is marked by a reorganization and growth of important brain regions including structures associating with reward seeking behaviors such as the nucleus accumbens (NA) and prefrontal cortex (PFC). These changes are impacted by stressors during the juvenile period and may lead to a predisposition to stress induced psychopathology and abnormal development of brain reward systems. Like in humans, adult rodents engage certain coping mechanisms such as increases in the consumption of calorie-rich palatable foods to reduce stress, but this behavior can lead to obesity and metabolic disorders. In this study, we examined whether stressors during the juvenile period led to increased caloric intake when a palatable diet was accessible, and whether this diet attenuated adult stress responses. In addition, we examined if the stress buffering effects produced by the palatable diet were also accompanied by an offset propensity towards obesity, and by alterations in mRNA expression of dopamine (DA) receptors in the NA and PFC in adulthood. To this end, juvenile male Wistar rats underwent episodic stressor exposure (forced swim, elevated platform stress and restraint) on postnatal days (PD) 27-29 and received access to regular chow or daily limited access to a palatable diet until adulthood. At the age of 2 months, rats were tested on a social interaction test that screens for anxiety-like behaviors and their endocrine responses to an acute stressor. Animals were sacrificed, and their brains processed to detect differences in DA receptor subtype expression in the PFC and NA using qPCR. Results showed that rats that were stressed during the juvenile period displayed higher social anxiety and a sensitized corticosterone response as adults and these effects were attenuated by access to the palatable diet. Nevertheless, rats that experienced juvenile stress and consumed a palatable diet showed greater adiposity in adulthood. Interestingly, the same group displayed greater mRNA expression of DA receptors at the NA. This suggests that access to a palatable diet mitigates the behavioral and endocrine effects of juvenile stressor exposure in adulthood, but at the cost of metabolic imbalances and a sensitized dopaminergic system.
Keywords: HPA-axis; dopamine receptors; juvenile stress; metabolic syndrome; nucleus accumbens; palatable food; prefrontal cortex; social interaction.
Figures



References
-
- Alsiö J., Olszewski P. K., Norbäck A. H., Gunnarsson Z. E. A., Levine A. S., Pickering C., et al. . (2010). Dopamine D1 receptor gene expression decreases in the nucleus accumbens upon long-term exposure to palatable food and differs depending on diet-induced obesity phenotype in rats. Neuroscience 171, 779–787. 10.1016/j.neuroscience.2010.09.046 - DOI - PubMed
-
- Arcego D. M., Krolow R., Lampert C., Noschang C., Ferreira A. G. K., Scherer E., et al. . (2014). Isolation during the prepubertal period associated with chronic access to palatable diets: effects on plasma lipid profile and liver oxidative stress. Physiol. Behav. 124, 23–32. 10.1016/j.physbeh.2013.10.029 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

