Redox-Mediated Mechanisms Fuel Monocyte Responses to CXCL12/HMGB1 in Active Rheumatoid Arthritis
- PMID: 30283452
- PMCID: PMC6157448
- DOI: 10.3389/fimmu.2018.02118
Redox-Mediated Mechanisms Fuel Monocyte Responses to CXCL12/HMGB1 in Active Rheumatoid Arthritis
Abstract
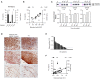
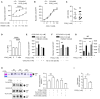
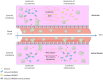
Chemokine synergy-inducing molecules are emerging as regulating factors in cell migration. The alarmin HMGB1, in its reduced form, can complex with CXCL12 enhancing its activity on monocytes via the chemokine receptor CXCR4, while the form containing a disulfide bond, by binding to TLR2 or TLR4, initiates a cascade of events leading to production of cytokines and chemokines. So far, the possibility that the CXCL12/HMGB1 heterocomplex could be maintained in chronic inflammation was debated, due to the release of reactive oxygen species. Therefore, we have assessed if the heterocomplex could remain active in Rheumatoid Arthritis (RA) and its relevance in the disease assessment. Monocytes from RA patients with active disease require a low concentration of HMGB1 to enhance CXCL12-induced migration, in comparison to monocytes from patients in clinical remission or healthy donors. The activity of the heterocomplex depends on disease activity, on the COX2 and JAK/STAT pathways, and is determined by the redox potential of the microenvironment. In RA, the presence of an active thioredoxin system correlates with the enhanced cell migration, and with the presence of the heterocomplex in the synovial fluid. The present study highlights how, in an unbalanced microenvironment, the activity of the thioredoxin system plays a crucial role in sustaining inflammation. Prostaglandin E2 stimulation of monocytes from healthy donors is sufficient to recapitulate the response observed in patients with active RA. The activation of mechanisms counteracting the oxidative stress in the extracellular compartment preserves HMGB1 in its reduced form, and contributes to fuel the influx of inflammatory cells. Targeting the heterocomplex formation and its activity could thus be an additional tool for dampening the inflammation sustained by cell recruitment, for those patients with chronic inflammatory conditions who poorly respond to current therapies.
Keywords: CXCL12; HMGB1; cell migration; monocytes; rheumatoid arthritis; thioredoxin.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

