Intrinsic Programming of Alveolar Macrophages for Protective Antifungal Innate Immunity Against Pneumocystis Infection
- PMID: 30283457
- PMCID: PMC6156154
- DOI: 10.3389/fimmu.2018.02131
Intrinsic Programming of Alveolar Macrophages for Protective Antifungal Innate Immunity Against Pneumocystis Infection
Abstract
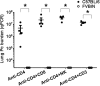
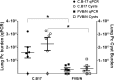
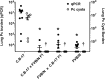
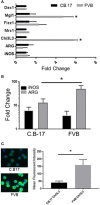
Invasive fungal infections, including Pneumocystis Pneumonia (PcP), remain frequent life-threatening conditions of patients with adaptive immune defects. While innate immunity helps control pathogen growth early during infection, it is typically not sufficient for complete protection against Pneumocystis and other human fungal pathogens. Alveolar macrophages (AM) possess pattern recognition molecules capable of recognizing antigenic and structural determinants of Pneumocystis. However, this pathogen effectively evades innate immunity to infect both immunocompetent and immunosuppressed hosts, albeit with differing outcomes. During our studies of mouse models of PcP, the FVB/N strain was identified as unique because of its ability to mount a protective innate immune response against Pneumocystis infection. In contrast to other immunocompetent strains, which become transiently infected prior to the onset of adaptive immunity, FVB/N mice rapidly eradicated Pneumocystis before an adaptive immune response was triggered. Furthermore, FVB/N mice remained highly resistant to infection even in the absence of functional T cells. The effector mechanism of innate protection required the action of functional alveolar macrophages, and the adoptive transfer of resistant FVB/N AMs, but not susceptible CB.17 AMs, conferred protection to immunodeficient mice. Macrophage IFNγ receptor signaling was not required for innate resistance, and FVB/N macrophages were found to display markers of alternative activation. IFNγ reprogrammed resistant FVB/N macrophages to a permissive M1 biased phenotype through a mechanism that required direct activation of the macrophage IFNγR. These results demonstrate that appropriately programmed macrophages provide protective innate immunity against this opportunistic fungal pathogen, and suggest that modulating macrophage function may represent a feasible therapeutic strategy to enhance antifungal host defense. The identification of resistant and susceptible macrophages provides a novel platform to study not only the mechanisms of macrophage-mediated antifungal defense, but also the mechanisms by which Pneumocystis evades innate immunity.
Keywords: alveolar macrophage; fungal pathogens; innate immunity; mouse models; pneumocystis.
Figures





Similar articles
-
Protective innate immunity against Pneumocystis does not require Stat6-dependent macrophage polarization.Infect Immun. 2024 Oct 15;92(10):e0022224. doi: 10.1128/iai.00222-24. Epub 2024 Aug 16. Infect Immun. 2024. PMID: 39150267 Free PMC article.
-
Conserved natural IgM antibodies mediate innate and adaptive immunity against the opportunistic fungus Pneumocystis murina.J Exp Med. 2010 Dec 20;207(13):2907-19. doi: 10.1084/jem.20100034. Epub 2010 Dec 13. J Exp Med. 2010. PMID: 21149550 Free PMC article.
-
Mitochondrial Reactive Oxygen Species Enhance Alveolar Macrophage Activity against Aspergillus fumigatus but Are Dispensable for Host Protection.mSphere. 2021 Jun 30;6(3):e0026021. doi: 10.1128/mSphere.00260-21. Epub 2021 Jun 2. mSphere. 2021. PMID: 34077261 Free PMC article.
-
Immune responses of different hosts to Pneumocystis infection.Eur Respir Rev. 2025 Jul 9;34(177):240247. doi: 10.1183/16000617.0247-2024. Print 2025 Jul. Eur Respir Rev. 2025. PMID: 40633972 Free PMC article. Review.
-
Innate immune memory of tissue-resident macrophages and trained innate immunity: Re-vamping vaccine concept and strategies.J Leukoc Biol. 2020 Sep;108(3):825-834. doi: 10.1002/JLB.4MR0220-446R. Epub 2020 Mar 3. J Leukoc Biol. 2020. PMID: 32125045 Review.
Cited by
-
Niflumic Acid Reverses Airway Mucus Excess and Improves Survival in the Rat Model of Steroid-Induced Pneumocystis Pneumonia.Front Microbiol. 2019 Jul 5;10:1522. doi: 10.3389/fmicb.2019.01522. eCollection 2019. Front Microbiol. 2019. PMID: 31333624 Free PMC article.
-
Immune Response in Pneumocystis Infections According to the Host Immune System Status.J Fungi (Basel). 2021 Jul 31;7(8):625. doi: 10.3390/jof7080625. J Fungi (Basel). 2021. PMID: 34436164 Free PMC article. Review.
-
The Dual Benefit of Sulfasalazine on Pneumocystis Pneumonia-Related Immunopathogenesis and Antifungal Host Defense Does Not Require IL-4Rα-Dependent Macrophage Polarization.Infect Immun. 2023 Apr 18;91(4):e0049022. doi: 10.1128/iai.00490-22. Epub 2023 Mar 14. Infect Immun. 2023. PMID: 36916933 Free PMC article.
-
Increased airway epithelial cell-derived exosomes activate macrophage-mediated allergic inflammation via CD100 shedding.J Cell Mol Med. 2021 Sep;25(18):8850-8862. doi: 10.1111/jcmm.16843. Epub 2021 Aug 20. J Cell Mol Med. 2021. PMID: 34414666 Free PMC article.
-
Pneumocystis Pneumonia: Pitfalls and Hindrances to Establishing a Reliable Animal Model.J Fungi (Basel). 2022 Jan 27;8(2):129. doi: 10.3390/jof8020129. J Fungi (Basel). 2022. PMID: 35205883 Free PMC article. Review.
References
-
- Kaplan JE, Hanson D, Dworkin MS, Frederick T, Bertolli J, Lindegren ML, et al. . Epidemiology of human immunodeficiency virus-associated opportunistic infections in the United States in the era of highly active antiretroviral therapy. Clin Infect Dis. (2000) 30(Suppl. 1):S5–14. 10.1086/313843 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

