Nematode-Infected Mice Acquire Resistance to Subsequent Infection With Unrelated Nematode by Inducing Highly Responsive Group 2 Innate Lymphoid Cells in the Lung
- PMID: 30283458
- PMCID: PMC6157322
- DOI: 10.3389/fimmu.2018.02132
Nematode-Infected Mice Acquire Resistance to Subsequent Infection With Unrelated Nematode by Inducing Highly Responsive Group 2 Innate Lymphoid Cells in the Lung
Abstract
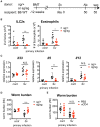
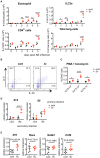
The immune responses against helminths have been investigated individually, and it is well-established that infected hosts develop an immunological memory to resist reinfection by the same pathogen. In contrast, it is poorly understood how the host immune system responds to subsequent infection by unrelated parasites after elimination of the first infection. We previously reported that infection of mice with Strongyloides venezuelensis induces the accumulation of group 2 innate lymphoid cells (ILC2s) in the lung. Here, we demonstrated that S. venezuelensis-experienced (Sv-exp) mice became significantly resistant against infection by Nippostrongylus brasiliensis. N. brasiliensis infection induced enhanced accumulation of ILC2s and eosinophils with increased expressions of mRNA for Th2 cytokines in the lungs of Sv-exp mice. The resistance was dependent on ILC2s, and eosinophils but not on CD4+ T cells. Furthermore, pulmonary ILC2s in Sv-exp mice acquired a highly responsive "trained" phenotype; in response to N. brasiliensis infection, they rapidly increased and produced IL-5 and IL-13, which in turn induced the early accumulation of eosinophils in the lungs. IL-33 was required for the accumulation of ILC2s and the resistance of mice against N. brasiliensis infection but insufficient for the induction of trained ILC2s. In conclusion, animals infected with one type of lung-migratory nematodes acquire a specific-antigen-independent resistance to another type of lung-migrating nematodes, providing animals with the capacity to protect against sequential infections with various lung-migratory nematodes.
Keywords: IL-33; eosinophils; innate immune memory; intestinal nematode; trained immunity.
Figures




References
-
- WHO Guidelines Approved by the Guidelines Review Committee (2017). Guideline: Preventive Chemotherapy to Control Soil-Transmitted Helminth Infections in At-Risk Population Groups. Geneva: WHO Guidelines Approved by the Guidelines Review Committee; (2017).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

