Durable Clinical Responses and Long-Term Follow-Up of Stage III-IV Non-Small-Cell Lung Cancer (NSCLC) Patients Treated With IDO Peptide Vaccine in a Phase I Study-A Brief Research Report
- PMID: 30283461
- PMCID: PMC6157336
- DOI: 10.3389/fimmu.2018.02145
Durable Clinical Responses and Long-Term Follow-Up of Stage III-IV Non-Small-Cell Lung Cancer (NSCLC) Patients Treated With IDO Peptide Vaccine in a Phase I Study-A Brief Research Report
Abstract
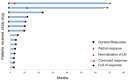
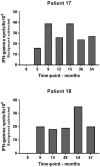
Background: Long-term follow-up on a clinical trial of 15 stage III-IV NSCLC patients treated with an Indoleamine 2,3-Dioxygenase (IDO) peptide vaccine (NCT01219348). Methods: Fifteen HLA-A2-positive patients with stable stage III-IV NSCLC after standard chemotherapy were treated with subcutaneous vaccinations (100 μg IDO5 peptide, sequence ALLEIASCL, formulated in 900 μl Montanide) biweekly for 2.5 months and thereafter monthly until progression or up to 5 years. Here we report long-term clinical follow-up, toxicity and immunity. Results: Three of 15 patients are still alive corresponding to a 6-year overall survival of 20 %. Two patients continued monthly vaccinations for 5 years (56 vaccines). One of the two patients developed a partial response (PR) of target lesions in the liver 15 months after the first vaccine and has remained in PR ever since. The other patient had a solitary distant metastasis in a lymph node in retroperitoneum at baseline which normalized during treatment. All following evaluation scans during the treatment have been tumor free. The vaccine was well tolerated for all 5 years with no long-term toxicities registered. The third long-term surviving patient discontinued vaccinations after 11 months due to disease progression. Flow cytometry analyses of PBMCs from the two long-term responders demonstrated stable CD8+ and CD4+ T-cell populations during treatment. In addition, presence of IDO-specific T-cells was detected by IFN-γ Elispot in both patients at several time points during treatment. Conclusion: IDO peptide vaccination was well tolerated for administration up to 5years. Two of 15 patients are long-term responders with ongoing clinical response 6 years after 1st vaccination.
Keywords: IDO; NSCLC; cancer; immunotherapy; peptide vaccine.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

