Proposed approach to the challenging management of progressive gastroesophageal reflux disease
- PMID: 30283600
- PMCID: PMC6162253
- DOI: 10.4253/wjge.v10.i9.175
Proposed approach to the challenging management of progressive gastroesophageal reflux disease
Abstract
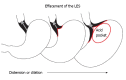
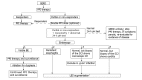
The progression of gastroesophageal reflux disease (GERD) in patients who are taking proton pump inhibitors (PPIs) has been reported by several investigators, leading to concerns that PPI therapy does not address all aspects of the disease. Patients who are at risk of progression need to be identified early in the course of their disease in order to receive preventive treatment. A review of the literature on GERD progression to Barrett's esophagus and the associated physiological and pathological changes was performed and risk factors for progression were identified. In addition, a potential approach to the prevention of progression is discussed. Current evidence shows that GERD can progress; however, patients at risk of progression may not be identified early enough for it to be prevented. Biopsies of the squamocolumnar junction that show microscopic intestinalization of metaplastic cardiac mucosa in endoscopically normal patients are predictive of future visible Barrett's esophagus, and an indicator of GERD progression. Such changes can be identified only through biopsy, which is not currently recommended for endoscopically normal patients. GERD treatment should aim to prevent progression. We propose that endoscopically normal patients who partially respond or do not respond to PPI therapy undergo routine biopsies at the squamocolumnar junction to identify histological changes that may predict future progression. This will allow earlier intervention, aimed at preventing Barrett's esophagus.
Keywords: Barrett’s esophagus; Endoscopy; Gastroesophageal reflux disease; Progression; Treatment.
Conflict of interest statement
Conflict-of-interest statement: Joachim Labenz has served as a consultant to EndoStim and Reckitt Benckiser, and has received honoraria for scientific presentations from AstraZeneca, EndoStim, Reckitt Benckiser, and Torax Medical Inc. Parakrama Chandrasoma has no conflict of interest. Laura Knapp is an employee of PharmaGenesis London, which received funding from EndoStim. Tom R DeMeester is currently a consultant to Torax Medical Inc. and has received honoraria for consultation and scientific presentation from EndoStim.
Figures


References
-
- Vakil N, van Zanten SV, Kahrilas P, Dent J, Jones R; Global Consensus Group. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol. 2006;101:1900–1920; quiz 1943. - PubMed
-
- DeMeester TR, Peters JH, Bremner CG, Chandrasoma P. Biology of gastroesophageal reflux disease: pathophysiology relating to medical and surgical treatment. Annu Rev Med. 1999;50:469–506. - PubMed
-
- Ronkainen J, Aro P, Storskrubb T, Johansson SE, Lind T, Bolling-Sternevald E, Graffner H, Vieth M, Stolte M, Engstrand L, et al. High prevalence of gastroesophageal reflux symptoms and esophagitis with or without symptoms in the general adult Swedish population: a Kalixanda study report. Scand J Gastroenterol. 2005;40:275–285. - PubMed
-
- Bytzer P, Jones R, Vakil N, Junghard O, Lind T, Wernersson B, Dent J. Limited ability of the proton-pump inhibitor test to identify patients with gastroesophageal reflux disease. Clin Gastroenterol Hepatol. 2012;10:1360–1366. - PubMed
-
- Vakil N. Review article: how valuable are proton-pump inhibitors in establishing a diagnosis of gastro-oesophageal reflux disease? Aliment Pharmacol Ther. 2005;22 Suppl 1:64–69. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

