Adverse drug reaction reporting in a large tertiary hospital in Saudi Arabia: results of an incentive strategy
- PMID: 30283626
- PMCID: PMC6166318
- DOI: 10.1177/2042098618790209
Adverse drug reaction reporting in a large tertiary hospital in Saudi Arabia: results of an incentive strategy
Abstract
Background: Underreporting is a common problem with spontaneous adverse drug reaction (ADR) reporting. In this study, we aim to describe the reporting of ADRs in a tertiary hospital and determine the effect of incentives to healthcare professionals on ADR reporting.
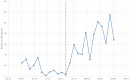
Methods: In this interventional study, a time series analysis was used to determine the effect of incentives on ADR reporting in a tertiary hospital between 2015 and 2016. The incentive strategy included public commendation of health care providers and nomination for a monthly award.
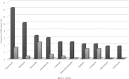
Results: A total of 967 ADRs were reported over a 2-year period. After the introduction of incentives in January 2016, the number of ADR reports per month increased by 40.6 (95% confidence interval: 26.1-55.1). The proportion of serious ADRs reported was significantly higher in 2016 (39/800) than 2015 (0/167) (p < 0.001). In 2016, there was a significant association between profession and serious ADR reporting (p < 0.001). A total of 14/21 ADRs (66.7%) reported by physicians in 2016 were serious compared with 20/700 (2.9%) reported by clinical pharmacists and 5/72 (6.9%) by nurses.
Conclusions: ADR reporting was improved by providing incentives, including commendation and reward, to healthcare professionals.
Keywords: Middle-East; drug safety; pharmacovigilance.
Conflict of interest statement
Conflict of interest statement: The authors declare that there is no conflict of interest.
Figures
References
-
- Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet 2000; 356: 1255–1259. - PubMed
-
- World Health Organisation. Pharmacovigilance, http://www.who.int/medicines/areas/quality_safety/safety_efficacy/pharmv... (2017, accessed 29 June 2018).
-
- Saudi Food and Drug Authority. Saudi Vigilance, http://www.sfda.gov.sa/en/drug/about/sector_departments/national_pharmac.... (2017, accessed 15 May 2018).
-
- Saudi Food and Drug Authority. Saudi Vigilance, https://www.sfda.gov.sa/en/drug/about/sector_departments/national_pharma... (2013, accessed 29 June 2018).
LinkOut - more resources
Full Text Sources
Research Materials