Tumor-Intrinsic PD-L1 Signaling in Cancer Initiation, Development and Treatment: Beyond Immune Evasion
- PMID: 30283733
- PMCID: PMC6156376
- DOI: 10.3389/fonc.2018.00386
Tumor-Intrinsic PD-L1 Signaling in Cancer Initiation, Development and Treatment: Beyond Immune Evasion
Abstract
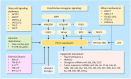
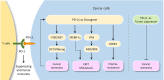
Although the role of PD-L1 in suppressing the anti-tumor immune response is extensively documented, recent discoveries indicate a distinct tumor-intrinsic role for PD-L1 in modulating epithelial-to-mesenchymal transition (EMT), cancer stem cell (CSC)-like phenotype, metastasis and resistance to therapy. In this review, we will focus on the newly discovered functions of PD-L1 in the regulation of cancer development, describe underlying molecular mechanisms responsible for PD-L1 upregulation and discuss current insights into novel components of PD-L1 signaling. Furthermore, we summarize our current understanding of the link between PD-L1 signaling and the EMT program as well as the CSC state. Tumor cell-intrinsic PD-L1 clearly contributes to cancer stemness, EMT, tumor invasion and chemoresistance in multiple tumor types. Conversely, activation of OCT4 signaling and upregulation of EMT inducer ZEB1 induce PD-L1 expression in cancer cells, thereby suggesting a possible immune evasion mechanism employed by cancer stem cells during metastasis. Our meta-analysis demonstrated that PD-L1 is co-amplified along with MYC, SOX2, N-cadherin and SNAI1 in the TCGA endometrial and ovarian cancer datasets. Further identification of immune-independent PD-L1 functions and characterization of crucial signaling events upstream or downstream of PD-L1 in diverse cancer types and specific cancer subtypes, would provide additional targets and new therapeutic approaches.
Keywords: CD274; EMT; PD-L1; cancer stem cells; metastasis; microRNA.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

