An autologous dendritic cell vaccine polarizes a Th-1 response which is tumoricidal to patient-derived breast cancer cells
- PMID: 30283982
- PMCID: PMC6326986
- DOI: 10.1007/s00262-018-2238-5
An autologous dendritic cell vaccine polarizes a Th-1 response which is tumoricidal to patient-derived breast cancer cells
Abstract
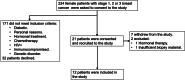
Breast cancer remains one of the leading causes of cancer-associated death worldwide. Conventional treatment is associated with substantial toxicity and suboptimal efficacy. We, therefore, developed and evaluated the in vitro efficacy of an autologous dendritic cell (DC) vaccine to treat breast cancer. We recruited 12 female patients with stage 1, 2, or 3 breast cancer and matured their DCs with autologous tumour-specific lysate, a toll-like receptor (TLR)-3 and 7/8 agonist, and an interferon-containing cocktail. The efficacy of the vaccine was evaluated by its ability to elicit a cytotoxic T-lymphocyte response to autologous breast cancer cells in vitro. Matured DCs (≥ 60% upregulation of CD80, CD86, CD83, and CCR7) produced high levels of the Th1 effector cytokine, IL12-p70 (1.2 ng/ml; p < 0.0001), compared to DCs pulsed with tumour lysate, or matured with an interferon-containing cocktail alone. We further showed that matured DCs enhance antigen-specific CD8 + T-cell responses to HER-2 (4.5%; p < 0.005) and MUC-1 (19%; p < 0.05) tetramers. The mature DCs could elicit a robust and dose-dependent antigen-specific cytotoxic T-lymphocyte response (65%) which was tumoricidal to autologous breast cancer cells in vitro compared to T-lymphocytes that were primed with autologous lysate loaded-DCs (p < 0.005). Lastly, we showed that the mature DCs post-cryopreservation maintained high viability, maintained their mature phenotype, and remained free of endotoxins or mycoplasma. We have developed a DC vaccine that is cytotoxic to autologous breast cancer cells in vitro. The tools and technology generated here will now be applied to a phase I/IIa clinical trial.
Keywords: Autologous primary breast cancer cells; Breast cancer; Dendritic cell vaccine; Immunotherapy; Tumour lysate.
Conflict of interest statement
The authors declare no potential conflict of interest.
Figures




Similar articles
-
Clinical scale electroloading of mature dendritic cells with melanoma whole tumor cell lysate is superior to conventional lysate co-incubation in triggering robust in vitro expansion of functional antigen-specific CTL.Int Immunopharmacol. 2013 Mar;15(3):488-97. doi: 10.1016/j.intimp.2013.01.009. Epub 2013 Feb 8. Int Immunopharmacol. 2013. PMID: 23474736
-
Tumor cell lysate-pulsed human dendritic cells induce a T-cell response against pancreatic carcinoma cells: an in vitro model for the assessment of tumor vaccines.Cancer Res. 2001 Sep 1;61(17):6445-50. Cancer Res. 2001. PMID: 11522639
-
Induction of cytolytic T lymphocytes against pediatric solid tumors in vitro using autologous dendritic cells pulsed with necrotic primary tumor.J Pediatr Surg. 2007 Jan;42(1):54-61; discussion 61. doi: 10.1016/j.jpedsurg.2006.09.008. J Pediatr Surg. 2007. PMID: 17208541
-
Dendritic cell-based combined immunotherapy with autologous tumor-pulsed dendritic cell vaccine and activated T cells for cancer patients: rationale, current progress, and perspectives.Hum Cell. 2003 Dec;16(4):175-82. doi: 10.1111/j.1749-0774.2003.tb00151.x. Hum Cell. 2003. PMID: 15147037 Review.
-
Tumor cell lysates as immunogenic sources for cancer vaccine design.Hum Vaccin Immunother. 2014;10(11):3261-9. doi: 10.4161/21645515.2014.982996. Hum Vaccin Immunother. 2014. PMID: 25625929 Free PMC article. Review.
Cited by
-
Stability Program in Dendritic Cell Vaccines: A "Real-World" Experience in the Immuno-Gene Therapy Factory of Romagna Cancer Center.Vaccines (Basel). 2022 Jun 23;10(7):999. doi: 10.3390/vaccines10070999. Vaccines (Basel). 2022. PMID: 35891165 Free PMC article.
-
Antitumour dendritic cell vaccination in a priming and boosting approach.Nat Rev Drug Discov. 2020 Sep;19(9):635-652. doi: 10.1038/s41573-020-0074-8. Epub 2020 Aug 6. Nat Rev Drug Discov. 2020. PMID: 32764681 Review.
-
Cancer stem cells: a target for overcoming therapeutic resistance and relapse.Cancer Biol Med. 2024 Feb 5;20(12):985-1020. doi: 10.20892/j.issn.2095-3941.2023.0333. Cancer Biol Med. 2024. PMID: 38164743 Free PMC article. Review.
-
Anti-Tumor Immunity to Patient-Derived Breast Cancer Cells by Vaccination with Interferon-Alpha-Conditioned Dendritic Cells (IFN-DC).Vaccines (Basel). 2024 Sep 17;12(9):1058. doi: 10.3390/vaccines12091058. Vaccines (Basel). 2024. PMID: 39340087 Free PMC article.
-
Immunotherapy in breast cancer: A clinician's perspective.J Natl Cancer Cent. 2021 Feb 24;1(2):47-57. doi: 10.1016/j.jncc.2021.01.001. eCollection 2021 Jun. J Natl Cancer Cent. 2021. PMID: 39035768 Free PMC article. Review.
References
-
- World Health Organisation (2015) Cancer Fact Sheet. http://www.who.int/mediacentre/factsheets/fs297/en/. Assessed 9 November 2016
-
- American Cancer Society (2017) Cancer Facts and Figures. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-.... Assessed 27 October 2017
-
- Breast cancer stages (2018) http://www.breastcancer.org/symptoms/diagnosis/staging. Assessed 5 June 2018
-
- Schadendorf D, Ugurel S, Schuler-Thurner B, et al. Dacarbazine (DTIC) versus vaccination with autologous peptide-pulsed dendritic cells (DC) in first-line treatment of patients with metastatic melanoma: a randomized phase III trial of the DC study group of the DeCOG. Ann Oncol. 2006;17:563–570. doi: 10.1093/annonc/mdj138. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

