2-Deoxy-D-ribose-5-phosphate aldolase (DERA): applications and modifications
- PMID: 30284013
- PMCID: PMC6244999
- DOI: 10.1007/s00253-018-9392-8
2-Deoxy-D-ribose-5-phosphate aldolase (DERA): applications and modifications
Abstract
2-Deoxy-D-ribose-5-phosphate aldolase (DERA) is a class I aldolase that offers access to several building blocks for organic synthesis. It catalyzes the stereoselective C-C bond formation between acetaldehyde and numerous other aldehydes. However, the practical application of DERA as a biocatalyst is limited by its poor tolerance towards industrially relevant concentrations of aldehydes, in particular acetaldehyde. Therefore, the development of proper experimental conditions, including protein engineering and/or immobilization on appropriate supports, is required. The present review is aimed to provide a brief overview of DERA, its history, and progress made in understanding the functioning of the enzyme. Furthermore, the current understanding regarding aldehyde resistance of DERA and the various optimizations carried out to modify this property are discussed.
Keywords: Aldol reaction; Aldolase; C–C bond; DERA; Immobilization; Protein engineering.
Conflict of interest statement
Conflict of interest
The authors declare that they have no conflict of interests.
Figures
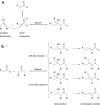


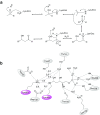



References
-
- Barbas CF, Wang YF, Wong CH. Deoxyribose-5-phosphate aldolase as a synthetic catalyst. J Am Chem Soc. 1990;112:2013–2014. doi: 10.1021/ja00161a064. - DOI
-
- Berger B, Faber K (1991) ‘Immunization’ of lipase against acetaldehyde emerging in acyl transfer reactions from vinyl acetate. J Chem Soc Chem Commun:1198–1200
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

