EXPRESS: Gremlin1 blocks vascular endothelial growth factor signalling in the pulmonary microvascular endothelium
- PMID: 30284507
- PMCID: PMC7066471
- DOI: 10.1177/2045894018807205
EXPRESS: Gremlin1 blocks vascular endothelial growth factor signalling in the pulmonary microvascular endothelium
Abstract
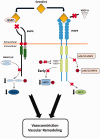
The bone morphogenetic protein (BMP) antagonist gremlin 1 plays a central role in the pathogenesis of hypoxic pulmonary hypertension (HPH). Recently, non-canonical functions of gremlin 1 have been identified, including specific binding to the vascular endothelial growth factor receptor-2 (VEGFR2). We tested the hypothesis that gremlin 1 modulates VEGFR2 signaling in the pulmonary microvascular endothelium.
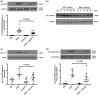
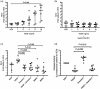
We examined the effect of gremlin 1 haploinsufficiency on the expression of VEGF responsive genes and proteins in the hypoxic (10% O2) murine lung in vivo. Using human microvascular endothelial cells in vitro we examined the effect of gremlin 1 on VEGF signaling.
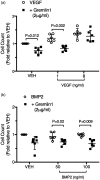
Gremlin 1 haploinsufficiency (Grem1+/–) attenuated the hypoxia-induced increase in gremlin 1 observed in the wild-type mouse lung. Reduced gremlin 1 expression in hypoxic Grem1+/– mice restored VEGFR2 expression and endothelial nitric oxide synthase (eNOS) expression and activity to normoxic values. Recombinant monomeric gremlin 1 inhibited VEGFA-induced VEGFR2 activation, downstream signaling, and VEGF-induced increases in Bcl-2, cell number, and the anti-apoptotic effect of VEGFA in vitro.
These results show that the monomeric form of gremlin 1 acts as an antagonist of VEGFR2 activation in the pulmonary microvascular endothelium. Given the previous demonstration that inhibition of VEGFR2 causes marked worsening of HPH, our results suggest that increased gremlin 1 in the hypoxic lung, in addition to blocking BMP receptor type-2 (BMPR2) signaling, contributes importantly to the development of PH by a non-canonical VEGFR2 blocking activity.
Figures

Similar articles
-
Nox1/Ref-1-mediated activation of CREB promotes Gremlin1-driven endothelial cell proliferation and migration.Redox Biol. 2019 Apr;22:101138. doi: 10.1016/j.redox.2019.101138. Epub 2019 Feb 8. Redox Biol. 2019. PMID: 30802716 Free PMC article.
-
Endothelial Nox1 oxidase assembly in human pulmonary arterial hypertension; driver of Gremlin1-mediated proliferation.Clin Sci (Lond). 2017 Jul 16;131(15):2019-2035. doi: 10.1042/CS20160812. Print 2017 Aug 1. Clin Sci (Lond). 2017. PMID: 28522681 Free PMC article.
-
Glioma cancer stem cells secrete Gremlin1 to promote their maintenance within the tumor hierarchy.Genes Dev. 2014 May 15;28(10):1085-100. doi: 10.1101/gad.235515.113. Epub 2014 May 1. Genes Dev. 2014. PMID: 24788093 Free PMC article.
-
Vascular endothelial injury assessed with functional techniques in systemic sclerosis patients with pulmonary arterial hypertension versus systemic sclerosis patients without pulmonary arterial hypertension: a systematic review and meta-analysis.Rheumatol Int. 2021 Jun;41(6):1045-1053. doi: 10.1007/s00296-021-04850-2. Epub 2021 Apr 8. Rheumatol Int. 2021. PMID: 33830321
-
Reversibility of endothelial dysfunction in diabetes: role of polyphenols.Br J Nutr. 2016 Jul;116(2):223-46. doi: 10.1017/S0007114516001884. Epub 2016 Jun 6. Br J Nutr. 2016. PMID: 27264638 Review.
Cited by
-
Gremlin: a complex molecule regulating wound healing and fibrosis.Cell Mol Life Sci. 2021 Dec;78(24):7917-7923. doi: 10.1007/s00018-021-03964-x. Epub 2021 Nov 3. Cell Mol Life Sci. 2021. PMID: 34731251 Free PMC article. Review.
-
Metabolic memory in diabetic foot syndrome (DFS): MICRO-RNAS, single nucleotide polymorphisms (SNPs) frequency and their relationship with indices of endothelial function and adipo-inflammatory dysfunction.Cardiovasc Diabetol. 2023 Jun 26;22(1):148. doi: 10.1186/s12933-023-01880-x. Cardiovasc Diabetol. 2023. PMID: 37365645 Free PMC article.
-
Expression of Osteopontin and Gremlin 1 Proteins in Cardiomyocytes in Ischemic Heart Failure.Int J Mol Sci. 2024 Jul 28;25(15):8240. doi: 10.3390/ijms25158240. Int J Mol Sci. 2024. PMID: 39125809 Free PMC article.
-
No evidence of Gremlin1-mediated activation of VEGFR2 signaling in endothelial cells.J Biol Chem. 2019 Nov 29;294(48):18041-18045. doi: 10.1074/jbc.AC119.010148. Epub 2019 Oct 11. J Biol Chem. 2019. PMID: 31604823 Free PMC article.
-
Insights into the Role of Gremlin-1, a Bone Morphogenic Protein Antagonist, in Cancer Initiation and Progression.Biomedicines. 2022 Jan 28;10(2):301. doi: 10.3390/biomedicines10020301. Biomedicines. 2022. PMID: 35203511 Free PMC article. Review.
References
-
- Seeger W, Adir Y, Barbera JA, et al. Pulmonary hypertension in chronic lung diseases. J Am Coll Cardiol 2013; 62: D109–116. - PubMed
-
- Naeije R. Pulmonary hypertension and right heart failure in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2005; 2: 20–22. - PubMed
-
- Yang K, Wang J, Lu W. Bone morphogenetic protein signalling in pulmonary hypertension: advances and therapeutic implications. Exp Physiol 2017; 102: 1083–1089. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

