Dapoxetine for the treatment of premature ejaculation: a meta-analysis of randomized controlled trials with trial sequential analysis
- PMID: 30284992
- PMCID: PMC6180218
- DOI: 10.5144/0256-4947.2018.366
Dapoxetine for the treatment of premature ejaculation: a meta-analysis of randomized controlled trials with trial sequential analysis
Abstract
Background: The safety and efficacy of dapoxetine for the treatment of premature ejaculation (PE) is still controversial. Thus, we decided to conduct a meta-analysis using trial sequential analysis (TSA) to determine the sufficiency of conclusions.
Objective: Evaluate the efficacy and safety of dapoxetine in the treatment of patients with PE and assess the reliability of the findings.
Design: Meta-analysis of randomized controlled trials (RCTs).
Methods: Electronic databases including PUBMED, EMBASE, Cochrane Library, CNKI and Wanfang data were reviewed up to July 2017. RCTs evaluating the efficacy of dapoxetine in patients with PE and reporting intravaginal ejaculatory latency time (IELT), patient global impression of change (PGIC) and/or adverse events (AEs) were included.
Main outcome measures: Mean differences between trials in efficacy for IELT, and risk ratios for PGIC and treatment-emergent AEs.
Sample: 8 RCTs.
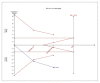
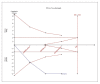
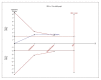
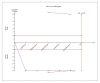
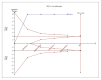
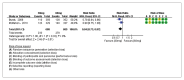
Results: For IELT and PGIC, significant effects were found for all doses of dapoxetine versus placebo, and similar results were obtained in subgroups of the 30-mg dose versus 60-mg dose. There were also statistically different dose-related effects on AEs. Trial sequential analysis showed that the result of our meta-analysis was confirmed and further trials are unnecessary.
Conclusions: The evidence suggests that dapoxetine may be a safe and effective drug for patients with PE.
Registration: Not registered, no published protocol.
Conflict of interest: No relationship with manufacturer of drug.
Conflict of interest statement
Figures

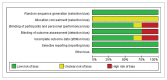



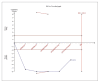
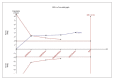
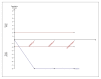




Similar articles
-
Dapoxetine for premature ejaculation: an updated meta-analysis of randomized controlled trials.Clin Ther. 2014 Dec 1;36(12):2003-2014. doi: 10.1016/j.clinthera.2014.09.011. Epub 2014 Oct 16. Clin Ther. 2014. PMID: 25438723
-
Efficacy of Dapoxetine for the treatment of premature ejaculation: a meta-analysis of randomized clinical trials on intravaginal ejaculatory latency time, patient-reported outcomes, and adverse events.Urology. 2015 Apr;85(4):856-61. doi: 10.1016/j.urology.2015.01.009. Urology. 2015. PMID: 25817107 Review.
-
Efficacy and safety of dapoxetine in men with premature ejaculation and concomitant erectile dysfunction treated with a phosphodiesterase type 5 inhibitor: randomized, placebo-controlled, phase III study.J Sex Med. 2013 Sep;10(9):2312-25. doi: 10.1111/jsm.12236. Epub 2013 Jul 11. J Sex Med. 2013. PMID: 23845016 Clinical Trial.
-
Efficacy and Safety of "On-Demand" Dapoxetine in Treatment of Patients with Premature Ejaculation: A Meta-Analysis.Med Sci Monit. 2019 Jun 7;25:4225-4232. doi: 10.12659/MSM.913606. Med Sci Monit. 2019. PMID: 31171764 Free PMC article.
-
The role of dapoxetine hydrochloride on-demand for the treatment of men with premature ejaculation.Sci Rep. 2014 Dec 1;4:7269. doi: 10.1038/srep07269. Sci Rep. 2014. PMID: 25434754 Free PMC article. Review.
Cited by
-
Selective dorsal neurotomy in the treatment of premature ejaculation: A protocol for systematic review and meta-analysis.Medicine (Baltimore). 2020 Aug 21;99(34):e21866. doi: 10.1097/MD.0000000000021866. Medicine (Baltimore). 2020. PMID: 32846840 Free PMC article.
-
Premature ejaculation - current concepts in the management: A narrative review.Int J Reprod Biomed. 2021 Jan 25;19(1):5-22. doi: 10.18502/ijrm.v19i1.8176. eCollection 2021 Jan. Int J Reprod Biomed. 2021. PMID: 33553999 Free PMC article. Review.
-
Premature Ejaculation: Aetiology and Treatment Strategies.Med Sci (Basel). 2019 Oct 25;7(11):102. doi: 10.3390/medsci7110102. Med Sci (Basel). 2019. PMID: 31731516 Free PMC article. Review.
-
Clinical Pharmacokinetic Evaluation of Optimized Liquisolid Tablets as a Potential Therapy for Male Sexual Dysfunction.Pharmaceutics. 2020 Dec 7;12(12):1187. doi: 10.3390/pharmaceutics12121187. Pharmaceutics. 2020. PMID: 33297307 Free PMC article.
-
Comparative study of on-demand and daily use of sertraline in treatment of premature ejaculation: A randomized clinical trial.Asian J Urol. 2021 Apr;8(2):209-214. doi: 10.1016/j.ajur.2019.10.002. Epub 2019 Oct 18. Asian J Urol. 2021. PMID: 33996478 Free PMC article.
References
-
- Bai Y, Pu C, Han P, Li J, Yuan H, Tang Y[7], et al. Selective serotonin reuptake inhibitors plus phosphodiesterase 5 inhibitors for premature ejaculation: a systematic review and meta-analysis. Urology. 2015 Oct;86(4):758–64. - PubMed
-
- Althof SE, McMahon CG, Waldinger MD, Serefoglu EC, Shindel AW, Adaikan PG, et al. An update of the International Society of Sexual Medicine’s guidelines for the diagnosis and treatment of premature ejaculation (PE) J Sex Med. 2014 Jun;11(6):1392–422. - PubMed
-
- Symonds T, Roblin D, Hart K, Althof S. How does premature ejaculation impact a man’s life? J Sex Marital Ther. 2003 Oct-Dec;29(5):361–70. - PubMed
-
- Althof SE, Abdo CH, Dean J, Hackett G, McCabe M, McMahon CG, et al. International Society for Sexual Medicine’s guidelines for the diagnosis and treatment of premature ejaculation. J Sex Med. 2010 Sep;7(9):2947–69. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

