The Clinical Utility of Point-of-Care Tests for Influenza in Ambulatory Care: A Systematic Review and Meta-analysis
- PMID: 30285232
- PMCID: PMC6579962
- DOI: 10.1093/cid/ciy837
The Clinical Utility of Point-of-Care Tests for Influenza in Ambulatory Care: A Systematic Review and Meta-analysis
Abstract
Background: Point-of-care tests (POCTs) for influenza are diagnostically superior to clinical diagnosis, but their impact on patient outcomes is unclear.
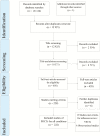
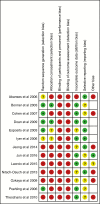
Methods: A systematic review of influenza POCTs versus usual care in ambulatory care settings. Studies were identified by searching six databases and assessed using the Cochrane risk of bias tool. Estimates of risk ratios (RR), standardised mean differences, 95% confidence intervals and I2 were obtained by random effects meta-analyses. We explored heterogeneity with sensitivity analyses and meta-regression.
Results: 12,928 citations were screened. Seven randomized studies (n = 4,324) and six non-randomized studies (n = 4,774) were included. Most evidence came from paediatric emergency departments. Risk of bias was moderate in randomized studies and higher in non-randomized studies. In randomized trials, POCTs had no effect on admissions (RR 0.93, 95% CI 0.61-1.42, I2 = 34%), returning for care (RR 1.00 95% CI = 0.77-1.29, I2 = 7%), or antibiotic prescribing (RR 0.97, 95% CI 0.82-1.15, I2 = 70%), but increased prescribing of antivirals (RR 2.65, 95% CI 1.95-3.60; I2 = 0%). Further testing was reduced for full blood counts (FBC) (RR 0.80, 95% CI 0.69-0.92 I2 = 0%), blood cultures (RR 0.82, 95% CI 0.68-0.99; I2 = 0%) and chest radiography (RR 0.81, 95% CI 0.68-0.96; I2 = 32%), but not urinalysis (RR 0.91, 95% CI 0.78-w1.07; I2 = 20%). Time in the emergency department was not changed. Fewer non-randomized studies reported these outcomes, with some findings reversed or attenuated (fewer antibiotic prescriptions and less urinalysis in tested patients).
Conclusions: Point-of-care testing for influenza influences prescribing and testing decisions, particularly for children in emergency departments. Observational evidence shows challenges for real-world implementation.
Keywords: diagnostics; influenza.
© The Author(s) 2018. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures



References
-
- World Health Organization. WHO public health research agenda for influenza. Public Health 2009; 1:1–18.
-
- Pitman RJ, Melegaro A, Gelb D, Siddiqui MR, Gay NJ, Edmunds WJ. Assessing the burden of influenza and other respiratory infections in England and Wales. J Infect 2007; 54:530–38. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

