Presynaptic loss of dynamin-related protein 1 impairs synaptic vesicle release and recycling at the mouse calyx of Held
- PMID: 30285293
- PMCID: PMC6292820
- DOI: 10.1113/JP276424
Presynaptic loss of dynamin-related protein 1 impairs synaptic vesicle release and recycling at the mouse calyx of Held
Abstract
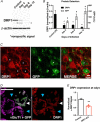
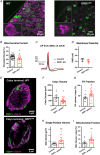
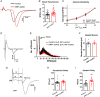
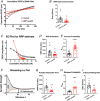
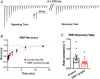
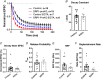
Key points: This study characterizes the mechanisms underlying defects in synaptic transmission when dynamin-related protein 1 (DRP1) is genetically eliminated. Viral-mediated knockout of DRP1 from the presynaptic terminal at the mouse calyx of Held increased initial release probability, reduced the size of the synaptic vesicle recycling pool and impaired synaptic vesicle recycling. Transmission defects could be partially restored by increasing the intracellular calcium buffering capacity with EGTA-AM, implying close coupling of Ca2+ channels to synaptic vesicles was compromised. Acute restoration of ATP to physiological levels in the presynaptic terminal did not reverse the synaptic defects. Loss of DRP1 impairs mitochondrial morphology in the presynaptic terminal, which in turn seems to arrest synaptic maturation.
Abstract: Impaired mitochondrial biogenesis and function is implicated in many neurodegenerative diseases, and likely affects synaptic neurotransmission prior to cellular loss. Dynamin-related protein 1 (DRP1) is essential for mitochondrial fission and is disrupted in neurodegenerative disease. In this study, we used the mouse calyx of Held synapse as a model to investigate the impact of presynaptic DRP1 loss on synaptic vesicle (SV) recycling and sustained neurotransmission. In vivo viral expression of Cre recombinase in ventral cochlear neurons of floxed-DRP1 mice generated a presynaptic-specific DRP1 knockout (DRP1-preKO), where the innervated postsynaptic cell was unperturbed. Confocal reconstruction of the calyx terminal suggested SV clusters and mitochondrial content were disrupted, and presynaptic terminal volume was decreased. Using postsynaptic voltage-clamp recordings, we found that DRP1-preKO synapses had larger evoked responses at low frequency stimulation. DRP1-preKO synapses also had profoundly altered short-term plasticity, due to defects in SV recycling. Readily releasable pool size, estimated with high-frequency trains, was dramatically reduced in DRP1-preKO synapses, suggesting an important role for DRP1 in maintenance of release-competent SVs at the presynaptic terminal. Presynaptic Ca2+ accumulation in the terminal was also enhanced in DRP1-preKO synapses. Synaptic transmission defects could be partially rescued with EGTA-AM, indicating close coupling of Ca2+ channels to SV distance normally found in mature terminals may be compromised by DRP1-preKO. Using paired recordings of the presynaptic and postsynaptic compartments, recycling defects could not be reversed by acute dialysis of ATP into the calyx terminals. Taken together, our results implicate a requirement for mitochondrial fission to coordinate postnatal synapse maturation.
Keywords: Adenosine Triphosphate; Animals; DNM1L protein, human; Mice; Mitochondrial Dynamics; Mitochondrial Proteins; Presynaptic Terminals; Synaptic Transmission.
© 2018 The Authors. The Journal of Physiology © 2018 The Physiological Society.
Figures





Similar articles
-
Ca2+ channel to synaptic vesicle distance accounts for the readily releasable pool kinetics at a functionally mature auditory synapse.J Neurosci. 2015 Feb 4;35(5):2083-100. doi: 10.1523/JNEUROSCI.2753-14.2015. J Neurosci. 2015. PMID: 25653365 Free PMC article.
-
Developmental shift to mitochondrial respiration for energetic support of sustained transmission during maturation at the calyx of Held.J Neurophysiol. 2021 Oct 1;126(4):976-996. doi: 10.1152/jn.00333.2021. Epub 2021 Aug 25. J Neurophysiol. 2021. PMID: 34432991 Free PMC article.
-
Activity-dependent changes in temporal components of neurotransmission at the juvenile mouse calyx of Held synapse.J Physiol. 2007 Jun 1;581(Pt 2):581-602. doi: 10.1113/jphysiol.2007.129833. Epub 2007 Mar 8. J Physiol. 2007. PMID: 17347264 Free PMC article.
-
Vesicle pools and short-term synaptic depression: lessons from a large synapse.Trends Neurosci. 2002 Apr;25(4):206-12. doi: 10.1016/s0166-2236(02)02139-2. Trends Neurosci. 2002. PMID: 11998689 Review.
-
Synaptic Vesicle Recycling at the Developing Presynapse.J Neurochem. 2025 Aug;169(8):e70206. doi: 10.1111/jnc.70206. J Neurochem. 2025. PMID: 40862509 Free PMC article. Review.
Cited by
-
Neuronal Glutamatergic Synaptic Clefts Alkalinize Rather Than Acidify during Neurotransmission.J Neurosci. 2020 Feb 19;40(8):1611-1624. doi: 10.1523/JNEUROSCI.1774-19.2020. Epub 2020 Jan 21. J Neurosci. 2020. PMID: 31964719 Free PMC article.
-
Effect of ApoE isoforms on mitochondria in Alzheimer disease.Neurology. 2020 Jun 9;94(23):e2404-e2411. doi: 10.1212/WNL.0000000000009582. Epub 2020 May 26. Neurology. 2020. PMID: 32457210 Free PMC article.
-
Elevated Biomarkers of Inflammation and Vascular Dysfunction Are Associated with Distal Sensory Polyneuropathy in People with HIV.Int J Mol Sci. 2024 Apr 11;25(8):4245. doi: 10.3390/ijms25084245. Int J Mol Sci. 2024. PMID: 38673830 Free PMC article.
-
Fenpropathrin induces degeneration of dopaminergic neurons via disruption of the mitochondrial quality control system.Cell Death Discov. 2020 Aug 25;6:78. doi: 10.1038/s41420-020-00313-y. eCollection 2020. Cell Death Discov. 2020. PMID: 32884840 Free PMC article.
-
NR4A1 promotes TNF‑α‑induced chondrocyte death and migration injury via activating the AMPK/Drp1/mitochondrial fission pathway.Int J Mol Med. 2020 Jan;45(1):151-161. doi: 10.3892/ijmm.2019.4398. Epub 2019 Nov 8. Int J Mol Med. 2020. Retraction in: Int J Mol Med. 2023 Sep;52(3):85. doi: 10.3892/ijmm.2023.5288. PMID: 31746366 Free PMC article. Retracted.
References
-
- Awatramani GB, Price GD & Trussell LO (2005). Modulation of transmitter release by presynaptic resting potential and background calcium levels. Neuron 48, 109–121. - PubMed
-
- Benard G, Bellance N, Jose C, Melser S, Nouette‐Gaulain K & Rossignol R (2010). Multi‐site control and regulation of mitochondrial energy production. Biochim Biophys Acta 1797, 698–709. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

