Dynamics of Quaternary Structure Transitions in R-State Carbonmonoxyhemoglobin Unveiled in Time-Resolved X-ray Scattering Patterns Following a Temperature Jump
- PMID: 30285440
- PMCID: PMC6580858
- DOI: 10.1021/acs.jpcb.8b07414
Dynamics of Quaternary Structure Transitions in R-State Carbonmonoxyhemoglobin Unveiled in Time-Resolved X-ray Scattering Patterns Following a Temperature Jump
Abstract
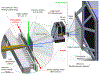
It is well-known that tetrameric hemoglobin binds ligands cooperatively by undergoing a ligand-induced T → R quaternary structure transition, a structure-function relationship that has long served as a model system for understanding allostery in proteins. However, kinetic studies of the reverse, R → T quaternary structure transition following photolysis of carbonmonoxyhemoglobin (HbCO) reveal complex behavior that may be better explained by the presence of two different R quaternary structures coexisting in thermal equilibrium. Indeed, we report here time-resolved small- and wide-angle X-ray scattering (SAXS/WAXS) patterns of HbCO following a temperature jump that not only provide unambiguous evidence for more than one R state, but also unveil the time scale for interconversion between them. Since the time scale for the photolysis-induced R → T transition is likely different for different R-states, this structural heterogeneity must be accounted for to properly explain the kinetic heterogeneity observed in time-resolved spectroscopic studies following photolysis of HbCO.
Conflict of interest statement
Notes
The authors declare no competing financial interest.
Figures
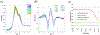


References
-
- Perutz MF Stereochemistry of Cooperative Effects in Haemoglobin. Nature 1970, 228, 726–739. - PubMed
-
- Monod J; Wyman J; Changeux JP On the Nature of Allosteric Transitions: A Plausible Model. J. Mol. Biol 1965, 12, 88–118. - PubMed
-
- Koshland DE Jr.; Nemethy G; Filmer D Comparison of Experimental Binding Data and Theoretical Models in Proteins Containing Subunits. Biochemistry 1966, 5, 365–385. - PubMed
-
- Szabo A; Karplus M A Mathematical Model for Structure-Function Relations in Hemoglobin. J. Mol. Biol 1972, 72, 163–197. - PubMed
-
- Yonetani T; Park SI; Tsuneshige A; Imai K; Kanaori K Global Allostery Model of Hemoglobin. Modulation of O2 Affinity, Cooperativity, and Bohr Effect by Heterotropic Allosteric Effectors. J. Biol. Chem 2002, 277, 34508–34520. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

