Oxygen versus air-driven nebulisers for exacerbations of chronic obstructive pulmonary disease: a randomised controlled trial
- PMID: 30285695
- PMCID: PMC6171193
- DOI: 10.1186/s12890-018-0720-7
Oxygen versus air-driven nebulisers for exacerbations of chronic obstructive pulmonary disease: a randomised controlled trial
Abstract
Background: In exacerbations of chronic obstructive pulmonary disease, administration of high concentrations of oxygen may cause hypercapnia and increase mortality compared with oxygen titrated, if required, to achieve an oxygen saturation of 88-92%. Optimally titrated oxygen regimens require two components: titrated supplemental oxygen to achieve the target oxygen saturation and, if required, bronchodilators delivered by air-driven nebulisation. The effect of repeated air vs oxygen-driven bronchodilator nebulisation in acute exacerbations of chronic obstructive pulmonary disease is unknown. We aimed to compare the effects of air versus oxygen-driven bronchodilator nebulisation on arterial carbon dioxide tension in exacerbations of chronic obstructive pulmonary disease.
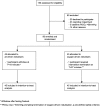
Methods: A parallel group double-blind randomised controlled trial in 90 hospital in-patients with an acute exacerbation of COPD. Participants were randomised to receive two 2.5 mg salbutamol nebulisers, both driven by air or oxygen at 8 L/min, each delivered over 15 min with a 5 min interval in-between. The primary outcome measure was the transcutaneous partial pressure of carbon dioxide at the end of the second nebulisation (35 min). The primary analysis used a mixed linear model with fixed effects of the baseline PtCO2, time, the randomised intervention, and a time by intervention interaction term; to estimate the difference between randomised treatments at 35 min. Analysis was by intention-to-treat.
Results: Oxygen-driven nebulisation was terminated in one participant after 27 min when the PtCO2 rose by > 10 mmHg, a predefined safety criterion. The mean (standard deviation) change in PtCO2 at 35 min was 3.4 (1.9) mmHg and 0.1 (1.4) mmHg in the oxygen and air groups respectively, difference (95% confidence interval) 3.3 mmHg (2.7 to 3.9), p < 0.001. The proportion of patients with a PtCO2 change ≥4 mmHg during the intervention was 18/45 (40%) and 0/44 (0%) for oxygen and air groups respectively.
Conclusions: Oxygen-driven nebulisation leads to an increase in PtCO2 in exacerbations of COPD. We propose that air-driven bronchodilator nebulisation is preferable to oxygen-driven nebulisation in exacerbations of COPD.
Trial registration: Australian New Zealand Clinical Trials Registry number ACTRN12615000389505 . Registration confirmed on 28/4/15.
Keywords: Air; Bronchodilator agents; Hypercapnia; Nebulisation; Oxygen.
Conflict of interest statement
Ethics approval and consent to participate
Ethics approval was obtained from the Health and Disability Ethics Committee, New Zealand (Reference 14/NTB/200). Written informed consent was obtained before any study-specific procedures.
Consent for publication
Not applicable.
Competing interests
All authors have completed the ICMJE uniform disclosure form at
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

