A longitudinal, multi-centre, superiority, randomized controlled trial of internet-based cognitive behavioural therapy (iCBT) versus treatment-as-usual (TAU) for negative experiences and posttraumatic stress following childbirth: the JUNO study protocol
- PMID: 30285758
- PMCID: PMC6167807
- DOI: 10.1186/s12884-018-1988-6
A longitudinal, multi-centre, superiority, randomized controlled trial of internet-based cognitive behavioural therapy (iCBT) versus treatment-as-usual (TAU) for negative experiences and posttraumatic stress following childbirth: the JUNO study protocol
Abstract
Background: About one-third of women report their childbirth as traumatic and up to 10% have severe traumatic stress responses to birth. The prevalence of Posttraumatic stress disorder following childbirth (PTSD FC) is estimated to 3%. Women with PTSD FC report the same symptoms as other patients with PTSD following other types of trauma. The effect of psychological treatment for women with PTSD FC has only been studied in a few trials. Similarly, studies on treatment needs for women not diagnosed as having PTSD FC but who nevertheless face psychological problems are lacking.
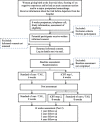
Methods/design: Women who rate their overall birth experience as negative on a Likert scale, and/or had an immediate caesarean section and/or a major postpartum haemorrhage are randomized to either internet delivered cognitive behaviour therapy (iCBT) plus treatment as usual (TAU) or TAU. The iCBT is to be delivered in two steps. The first step consists of six weekly modules for both the woman and her partner (if they wish to participate) with minimal therapeutic support. Step 2 consists of eight weekly modules with extended therapeutic support and will be offered to participants whom after step 1 report PTSD FC. Assessments will be made at baseline, 6 weeks, 14 weeks, and at follow-ups at 1, 2, 3 and 4 years after baseline. The primary outcome measures are symptoms of posttraumatic stress and depression. Secondary outcomes are quality of life, parent-child bonding, marital satisfaction, coping strategies, experience regarding the quality of care received, health-related quality of life, number of re-visits to the clinic and number of appointments for counselling during the 4 years' period after the negative childbirth experience, time until the woman gets pregnant again, and the type of birth in the subsequent pregnancy. A health economic evaluation in the form of a cost utility analysis will be conducted.
Discussion: This study protocol describes a randomized controlled trial that will provide information about the effectiveness of iCBT in women with negative experiences, posttraumatic stress, and PTSD FC.
Trial registration: ISRCTN39318241 . Date for registration 12/01/2017, retrospectively registered.
Keywords: Immediate caesarean section; Negative birth experience; PTSD; PTSD following childbirth; Postpartum haemorrhage; Posttraumatic stress following childbirth; Study protocol; iCBT.
Conflict of interest statement
Ethics approval and consent to participate
The Regional Ethics Review Board in Uppsala has approved the trial including all the participating sites (2012/495/1). All participants in this research provided their written consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- Socialstyrelsen . Graviditeter, förlossningar och nyfödda barn: medicinska födelseregistret 1973–2014 : assisterad befruktning 1991–2013. Stockholm: Socialstyrelsen; 2015.
-
- de Graaff Lisanne F., Honig Adriaan, van Pampus Mariëlle G., Stramrood Claire A.I. Preventing post-traumatic stress disorder following childbirth and traumatic birth experiences: a systematic review. Acta Obstetricia et Gynecologica Scandinavica. 2018;97(6):648–656. doi: 10.1111/aogs.13291. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

