Efficacy of anti-PD-1/PD-L1 antibodies after discontinuation due to adverse events in non-small cell lung cancer patients (HANSHIN 0316)
- PMID: 30285770
- PMCID: PMC6171229
- DOI: 10.1186/s12885-018-4819-2
Efficacy of anti-PD-1/PD-L1 antibodies after discontinuation due to adverse events in non-small cell lung cancer patients (HANSHIN 0316)
Abstract
Background: Immune checkpoint inhibitors (ICIs) have emerged as promising therapeutic agents in non-small cell lung cancer (NSCLC). However, the duration for which ICIs should be continued remains a clinical problem.
Methods: We examined the efficacy of anti-PD-1/PD-L1 inhibitors after the discontinuation of antibodies due to adverse events (AEs) in patients with NSCLC. This was a multicenter retrospective study that analyzed NSCLC patients who were treated with PD-1/PD-L1 inhibitors by August 2016.
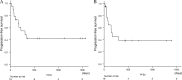
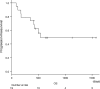
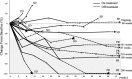
Results: The patients with NSCLC were 18 males and 1 female at a median 67 years of age (range: 49-80 years). Eighteen of 19 patients were treated with nivolumab, one was with atezolizumab. Approximately half of AEs were interstitial pneumonia. Fourteen patients (73.7%) were treated with steroid therapy. The median number of treatment cycles was 7 (range, 1-70), and the median duration of treatment was 2.8 months (range, 1 day-32.9 months). The overall response rate with confirmation during treatment was 21.1%. The median progression-free survival (PFS) was 10.2 months (95% confidence interval [CI] = 3.2-17.1 months) and 5.6 months (95% CI = 0-12.2 months) from the initiation and the discontinuation of PD-1/PD-L1 treatment, respectively. The median PFS after discontinuation according to the confirmed response during administration was not reached for partial response (PR) and 4.9 months (95% CI, 3.7-6.0) for stable disease (SD) patients (P = 0.02).
Conclusion: The PFS of the PR patients was completely different from that of the SD patients. The cases with PR prior to the onset of AE tended to show a durable response after the discontinuation of PD-1/PD-L1 inhibitors.
Keywords: Adverse event; Anti-PD-1/L1 inhibitor; Discontinuation; Non-small cell lung cancer.
Conflict of interest statement
Ethics approval and consent to participate
This research conformed to the provisions of the Declaration of Helsinki. This retrospective study was approved by the ethics committee of Kobe University (approval No.160122) and each institutions. Consent to participate: this is a retrospective study and consent to participate was waived by the Ethics Committee of Kobe University and each institution.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

Similar articles
-
FDA analyses of survival in older adults with metastatic non-small cell lung cancer in controlled trials of PD-1/PD-L1 blocking antibodies.Semin Oncol. 2018 Aug;45(4):220-225. doi: 10.1053/j.seminoncol.2018.08.007. Epub 2018 Oct 31. Semin Oncol. 2018. PMID: 30391014 Review.
-
Safety and Efficacy of PD-1/PD-L1 Inhibitors in Treatment-Naive and Chemotherapy-Refractory Patients With Non-Small-Cell Lung Cancer: A Systematic Review and Meta-Analysis.Clin Lung Cancer. 2018 May;19(3):e335-e348. doi: 10.1016/j.cllc.2018.01.002. Epub 2018 Jan 10. Clin Lung Cancer. 2018. PMID: 29433902
-
The impact of high PD-L1 expression on the surrogate endpoints and clinical outcomes of anti-PD-1/PD-L1 antibodies in non-small cell lung cancer.Lung Cancer. 2019 Feb;128:113-119. doi: 10.1016/j.lungcan.2018.12.023. Epub 2018 Dec 26. Lung Cancer. 2019. PMID: 30642442
-
The efficacy and safety of anti-PD-1/PD-L1 antibodies combined with chemotherapy or CTLA4 antibody as a first-line treatment for advanced lung cancer.Int J Cancer. 2018 Jun 1;142(11):2344-2354. doi: 10.1002/ijc.31252. Epub 2018 Jan 25. Int J Cancer. 2018. PMID: 29318609
-
Early serum tumor marker dynamics predict progression-free and overall survival in single PD-1/PD-L1 inhibitor treated advanced NSCLC-A retrospective cohort study.Lung Cancer. 2019 Aug;134:59-65. doi: 10.1016/j.lungcan.2019.05.033. Epub 2019 May 31. Lung Cancer. 2019. PMID: 31319996
Cited by
-
Long-term complete remission and peripheral biomarkers in Hodgkin lymphoma patients after decitabine-plus-camrelizumab epi-immunotherapy and treatment cessation.MedComm (2020). 2023 Nov 22;4(6):e428. doi: 10.1002/mco2.428. eCollection 2023 Dec. MedComm (2020). 2023. PMID: 38020717 Free PMC article.
-
Circulating Tumor DNA Monitoring Reveals Molecular Progression before Radiologic Progression in a Real-life Cohort of Patients with Advanced Non-small Cell Lung Cancer.Cancer Res Commun. 2022 Oct 13;2(10):1174-1187. doi: 10.1158/2767-9764.CRC-22-0258. eCollection 2022 Oct. Cancer Res Commun. 2022. PMID: 36969747 Free PMC article. Clinical Trial.
-
Prognostic significance of the radiologic features of pneumonitis induced by anti-PD-1 therapy.Cancer Med. 2020 May;9(9):3070-3077. doi: 10.1002/cam4.2974. Epub 2020 Mar 9. Cancer Med. 2020. PMID: 32150668 Free PMC article.
-
Knowledge Gaps and Research Priorities in Immune Checkpoint Inhibitor-related Pneumonitis. An Official American Thoracic Society Research Statement.Am J Respir Crit Care Med. 2019 Sep 15;200(6):e31-e43. doi: 10.1164/rccm.201906-1202ST. Am J Respir Crit Care Med. 2019. PMID: 31518182 Free PMC article.
-
Evaluation of 124I-JS001 for hPD1 immuno-PET imaging using sarcoma cell homografts in humanized mice.Acta Pharm Sin B. 2020 Jul;10(7):1321-1330. doi: 10.1016/j.apsb.2020.02.004. Epub 2020 Feb 19. Acta Pharm Sin B. 2020. PMID: 32874831 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

