Satisfactory virological response and fibrosis improvement of sofosbuvir-based regimens for Chinese patients with hepatitis C virus genotype 3 infection: results of a real-world cohort study
- PMID: 30285800
- PMCID: PMC6167801
- DOI: 10.1186/s12985-018-1066-8
Satisfactory virological response and fibrosis improvement of sofosbuvir-based regimens for Chinese patients with hepatitis C virus genotype 3 infection: results of a real-world cohort study
Abstract
Background: Chronic hepatitis C virus (HCV) genotype (GT) 3 infection with advanced liver disease has emerged as a challenging to treat by direct-acting antivirals (DAAs), but the efficacy of DAAs in Chinese HCV-GT3 patients is rarely reported. This study aimed to analyze the efficacy of sofosbuvir (SOF)-based regimens in Chinese patients with HCV-GT3 and compensated liver disease.
Methods: This was a registered retrospective study. All patients had completed at least 12 weeks SOF-based regimens therapy (with or without RBV), and were followed up for at least 24 weeks after therapy discontinuation. The primary endpoint was sustained virological response 24 weeks after end of therapy (SVR24).
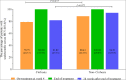
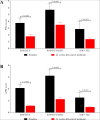
Results: A total of 102 patients who completed at least 12 weeks therapy were finally included, with 57 in SOF + Daclatasvir (SOF + DCV), 24 in SOF + DCV + ribavirin (RBV) and 21 in SOF/Velpatasvir (SOF/VEL). The total SVR24 rate was achieved in 90.20% (92/102), with 85.96% (49/57) in SOF + DCV, 91.67% (22/24) in SOF + DCV + RBV and 100.00% (21/21) in SOF/VEL. Among 10 relapsed patients (8 in SOF + DCV and 2 in SOF + DCV + RBV), the short course (12 weeks) of therapy and no RBV addition may be the leading cause. In this cohort, the SVR24 rate was not statistically different between patients with and without cirrhosis (81.82% [27/33] vs. 94.20% [65/69], P = 0.073). Additionally, both FIB-4 (4.03 vs. 2.08, P < 0.001) and APRI (2.15 vs. 0.68, P < 0.001) scores were significant improved from baseline to week 24 after completion of therapy, regardless of the presence of cirrhosis.
Conclusion: SOF-based regimens are highly effective in viral clearance and fibrosis remission for Chinese patients with HCV-GT3 infection. If available, SOF/VEL should be first considered.
Keywords: Chronic hepatitis C; Direct-acting antivirals; Genotype 3; Hepatitis C virus; Sofosbuvir-based regimens.
Conflict of interest statement
Ethics approval and consent to participate
This study was approved by the Ethics Committee of West China Hospital, Sichuan University.
Consent for publication
All authors approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures




Similar articles
-
Real-world effectiveness of daclatasvir plus sofosbuvir and velpatasvir/sofosbuvir in hepatitis C genotype 2 and 3.J Hepatol. 2019 Jan;70(1):15-23. doi: 10.1016/j.jhep.2018.09.018. Epub 2018 Sep 26. J Hepatol. 2019. PMID: 30266283
-
Real-world effectiveness of direct-acting antiviral agents for chronic hepatitis C patients with genotype-2 infection after completed treatment.Kaohsiung J Med Sci. 2021 Apr;37(4):334-345. doi: 10.1002/kjm2.12315. Epub 2020 Nov 5. Kaohsiung J Med Sci. 2021. PMID: 33151016 Free PMC article.
-
High sustained virologic response in genotypes 3 and 6 with generic NS5A inhibitor and sofosbuvir regimens in chronic HCV in myanmar.J Viral Hepat. 2019 Oct;26(10):1186-1199. doi: 10.1111/jvh.13133. Epub 2019 Jun 10. J Viral Hepat. 2019. PMID: 31104344
-
Real-World Effectiveness of Direct-Acting Antiviral Regimens against Hepatitis C Virus (HCV) Genotype 3 Infection: A Systematic Review and Meta-Analysis.Ann Hepatol. 2021 Jul-Aug;23:100268. doi: 10.1016/j.aohep.2020.09.012. Epub 2020 Oct 12. Ann Hepatol. 2021. PMID: 33059055
-
Daclatasvir and Sofosbuvir Versus Sofosbuvir and Ribavirin in Patients with Chronic Hepatitis C Coinfected with HIV: A Matching-adjusted Indirect Comparison.Clin Ther. 2016 Feb;38(2):404-12. doi: 10.1016/j.clinthera.2015.12.017. Epub 2016 Feb 3. Clin Ther. 2016. PMID: 26839044
Cited by
-
Hepatitis C Guidance 2019 Update: American Association for the Study of Liver Diseases-Infectious Diseases Society of America Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection.Hepatology. 2020 Feb;71(2):686-721. doi: 10.1002/hep.31060. Hepatology. 2020. PMID: 31816111 Free PMC article. No abstract available.
-
Efficacy of 12-weeks velpatasvir plus sofosbuvir-based regimen in HCV-naive subjects with mild fibrosis: a meta-analysis.Acta Biomed. 2019 May 23;90(2):187-196. doi: 10.23750/abm.v90i2.8374. Acta Biomed. 2019. PMID: 31124995 Free PMC article.
-
Global real-world evidence of sofosbuvir/velpatasvir as simple, effective HCV treatment: Analysis of 5552 patients from 12 cohorts.Liver Int. 2020 Aug;40(8):1841-1852. doi: 10.1111/liv.14537. Epub 2020 Jun 9. Liver Int. 2020. PMID: 32449966 Free PMC article.
-
Sofosbuvir-Based Therapies Achieved Satisfactory Virological Response in Chinese Individuals with Genotypes 3 and 6 Infections: A Real-World Experience.Infect Drug Resist. 2021 Jun 21;14:2297-2307. doi: 10.2147/IDR.S312902. eCollection 2021. Infect Drug Resist. 2021. PMID: 34188496 Free PMC article.
-
Efficacy and safety of sofosbuvir-based pangenotypic direct-acting antiviral agents for chronic hepatitis C patients without genotype determination: Real-world experience of a retrospective study.Medicine (Baltimore). 2020 Oct 23;99(43):e22726. doi: 10.1097/MD.0000000000022726. Medicine (Baltimore). 2020. PMID: 33120769 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

