A novel model to correlate hydrogel spacer placement, perirectal space creation, and rectum dosimetry in prostate stereotactic body radiotherapy
- PMID: 30285812
- PMCID: PMC6167802
- DOI: 10.1186/s13014-018-1135-6
A novel model to correlate hydrogel spacer placement, perirectal space creation, and rectum dosimetry in prostate stereotactic body radiotherapy
Abstract
Background: The SpaceOAR hydrogel is employed to limit rectal radiation dose during prostate radiotherapy. We identified a novel parameter - the product of angle θ and hydrogel volume - to quantify hydrogel placement. This parameter predicted rectum dosimetry and acute rectal toxicity in prostate cancer patients treated with stereotactic body radiotherapy to 36.25 Gy in 5 fractions.
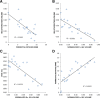
Methods: Twenty men with low- and intermediate-risk prostate cancer underwent hydrogel placement from 2015 to 2017. Hydrogel symmetry was assessed on the CT simulation scan in 3 axial slices (midgland, 1 cm above midgland, 1 cm below midgland). Two novel parameters quantifying hydrogel placement - hydrogel volume and angle θ formed by the prostate, hydrogel, and rectum - were measured, and the normalized product of θ and hydrogel volume calculated. These were then correlated with perirectal distance, rectum maximum 1-3 cc point doses (rDmax 1-3 cc), and rectum volumes receiving 80-95% of the prescription dose (rV80-95%). Acute rectal toxicity was recorded per RTOG criteria.
Results: In 50% of patients, hydrogel placement was symmetric bilaterally to within 1 cm of midline in all three CT simulation scan axial slices. Lateral hydrogel asymmetry < 2 cm in any one axial slice did not affect rectum dosimetry, but absence of hydrogel in the inferior axial slice resulted in a mean increase of 171 cGy in the rDmax 1 cc (p < 0.005). The perirectal distance measured at prostate midgland, midline (mean 9.1 ± 4.3 mm) correlated strongly with rV95 (R2 0.6, p < 0.001). The mean hydrogel volume and θ were 10.3 ± 4.5 cc and 70 ± 49°, respectively. Perirectal distance, rV95 and rDmax 1 cc correlated with hydrogel angle θ (p < 0.01), and yet more strongly with the novel metric θ*hydrogel volume (p < 0.001). With a median follow up of 14 months, no rectal toxicity >grade 2 was observed. Low grade rectal toxicity was observed in a third of men and resolved within 1 month of SBRT. Men who had these symptoms had higher rDmax 1 cc and smaller θ*hydrogel volume measurements.
Conclusions: Optimal hydrogel placement occurs at prostate midgland, midline. The novel parameter θ*hydrogel volume describes a large proportion of rectum dosimetric benefit derived from hydrogel placement, and can be used to assess the learning curve phenomenon for hydrogel placement.
Keywords: Dosimetry; Prostate cancer; Rectal toxicity; SpaceOAR hydrogel; Stereotactic body radiotherapy.
Conflict of interest statement
Ethics approval and consent to participate
Retrospective chart review of patients with prostate cancer was conducted from our single-institution IRB-approved database # AAAQ8136.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



Similar articles
-
Stereotactic body radiotherapy with periprostatic hydrogel spacer for localized prostate cancer: toxicity profile and early oncologic outcomes.Radiat Oncol. 2019 Aug 2;14(1):136. doi: 10.1186/s13014-019-1346-5. Radiat Oncol. 2019. PMID: 31375119 Free PMC article. Clinical Trial.
-
SpaceOAR hydrogel spacer injection prior to stereotactic body radiation therapy for men with localized prostate cancer: A systematic review.Medicine (Baltimore). 2021 Dec 10;100(49):e28111. doi: 10.1097/MD.0000000000028111. Medicine (Baltimore). 2021. PMID: 34889268 Free PMC article.
-
Hydrogel Spacer Prospective Multicenter Randomized Controlled Pivotal Trial: Dosimetric and Clinical Effects of Perirectal Spacer Application in Men Undergoing Prostate Image Guided Intensity Modulated Radiation Therapy.Int J Radiat Oncol Biol Phys. 2015 Aug 1;92(5):971-977. doi: 10.1016/j.ijrobp.2015.04.030. Epub 2015 Apr 23. Int J Radiat Oncol Biol Phys. 2015. PMID: 26054865 Clinical Trial.
-
Hydrogel spacer distribution within the perirectal space in patients undergoing radiotherapy for prostate cancer: Impact of spacer symmetry on rectal dose reduction and the clinical consequences of hydrogel infiltration into the rectal wall.Pract Radiat Oncol. 2017 May-Jun;7(3):195-202. doi: 10.1016/j.prro.2016.10.004. Epub 2016 Oct 17. Pract Radiat Oncol. 2017. PMID: 28089528 Clinical Trial.
-
Association of the Placement of a Perirectal Hydrogel Spacer With the Clinical Outcomes of Men Receiving Radiotherapy for Prostate Cancer: A Systematic Review and Meta-analysis.JAMA Netw Open. 2020 Jun 1;3(6):e208221. doi: 10.1001/jamanetworkopen.2020.8221. JAMA Netw Open. 2020. PMID: 32585020 Free PMC article.
Cited by
-
Relationship between hydrogel spacer distribution and dosimetric parameters in linear-accelerator-based stereotactic body radiotherapy for prostate cancer.J Appl Clin Med Phys. 2024 Jun;25(6):e14294. doi: 10.1002/acm2.14294. Epub 2024 Feb 6. J Appl Clin Med Phys. 2024. PMID: 38319652 Free PMC article.
-
Stereotactic body radiotherapy with periprostatic hydrogel spacer for localized prostate cancer: toxicity profile and early oncologic outcomes.Radiat Oncol. 2019 Aug 2;14(1):136. doi: 10.1186/s13014-019-1346-5. Radiat Oncol. 2019. PMID: 31375119 Free PMC article. Clinical Trial.
-
Chronological changes of lower urinary tract symptoms after low-dose-rate brachytherapy for prostate cancer using SpaceOAR® system.Prostate Int. 2022 Dec;10(4):207-212. doi: 10.1016/j.prnil.2022.06.003. Epub 2022 Jun 29. Prostate Int. 2022. PMID: 36570644 Free PMC article.
-
SpaceOAR hydrogel spacer injection prior to stereotactic body radiation therapy for men with localized prostate cancer: A systematic review.Medicine (Baltimore). 2021 Dec 10;100(49):e28111. doi: 10.1097/MD.0000000000028111. Medicine (Baltimore). 2021. PMID: 34889268 Free PMC article.
-
Hydrogel technologies in andrology: advances in research and prospective applications.Front Pharmacol. 2025 Jul 28;16:1635007. doi: 10.3389/fphar.2025.1635007. eCollection 2025. Front Pharmacol. 2025. PMID: 40792211 Free PMC article. Review.
References
-
- Lukka H., Stephanie P., Bruner D., Bahary J.P., Lawton C.A.F., Efstathiou J.A., Kudchadker R., Ponsky L., Seaward S.A., Dayes I.S., Gopaul D.D., Michalski J.M., Delouya G., Kaplan I.D., Horwitz E.M., Roach M., Pinover W.H., Beyer D.C., Sandler H.M., Kachnic L.A. Patient-Reported Outcomes in NRG Oncology/RTOG 0938, a Randomized Phase 2 Study Evaluating 2 Ultrahypofractionated Regimens (UHRs) for Prostate Cancer. International Journal of Radiation Oncology*Biology*Physics. 2016;94(1):2. doi: 10.1016/j.ijrobp.2015.10.046. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

