Outcome measures in clinical trials of treatments for acute severe haemorrhage
- PMID: 30285839
- PMCID: PMC6167881
- DOI: 10.1186/s13063-018-2900-4
Outcome measures in clinical trials of treatments for acute severe haemorrhage
Abstract
Background: Acute severe haemorrhage is a common complication of injury, childbirth, surgery, gastrointestinal pathologies and other medical conditions. Bleeding is a major cause of death, but patients also die from non-bleeding causes, the frequency of which varies by the site of haemorrhage and between populations. Because patients can bleed to death within hours, established interventions inevitably take priority over randomisation into a trial. These circumstances raise challenges in selecting appropriate outcome measures for clinical trials of haemostatic interventions.
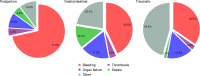
Main body: We use data from three large randomised controlled trials in acute severe haemorrhage (CRASH-2, WOMAN and HALT-IT) to explore the strengths and limitations of outcome measures commonly used in trials of haemostatic treatments, including all-cause and cause-specific mortality, blood transfusion and surgical interventions. Many deaths following acute severe haemorrhage are due to patient comorbidities or complications rather than bleeding. If non-bleeding deaths are unaffected by a haemostatic intervention, even large trials will have low power to detect an effect on all-cause mortality. Due to the dilution from deaths unaffected or reduced by the trial treatment, all-cause mortality can also obscure important harmful effects. Additionally, because the relative contributions of different causes of death vary within and between patient populations, all-cause mortality is not generalisable. Different causes of death occur at different time intervals from bleeding onset, with bleeding deaths generally occurring early. Time-specific mortality can therefore be used as a proxy for cause in un-blinded trials where bias is a concern or in situations where cause of death cannot be assessed. Urgent treatment is critical, and so post-randomisation blood transfusion and surgery are often planned before or at the time of randomisation and therefore cannot be influenced by the trial treatment.
Conclusions: All-cause mortality has low power, lacks generalisability and can obscure harmful effects. Cause-specific mortality, such as death due to bleeding or thrombosis, avoids these drawbacks. In certain scenarios, time-specific mortality can be used as a proxy for cause-specific mortality. Blood transfusion and surgical procedures have limited utility as outcome measures in trials of haemostatic treatments.
Keywords: Blood transfusion; Clinical trial; Haemorrhage; Haemostasis; Mortality; Outcome measure; Trial methodology.
Conflict of interest statement
Ethics approval and consent to participate
We conducted the CRASH-2, WOMAN and HALT-IT trials in accordance with good clinical practice guidelines. The relevant ethics committees and regulatory agencies approved the consent procedures. We obtained informed consent from the patient if physical and mental capacity allowed. If a person could not give consent, we obtained proxy consent from a relative or representative. If no proxy was available, then if local regulation allowed, we deferred or waived the consent. In these cases, we told the patient about the trial as soon as possible and obtained consent for use of the data collected.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
-
- Parry Smith WR, Gallos ID, Williams HM, Widmer M, Angolkar M, Tobias A, et al. First-line uterotonics for treating postpartum haemorrhage: a systematic review and network meta-analysis. Cochrane Database Syst Rev. 2017;8:CD012754.
-
- CRASH-2 trial collaborators. Shakur H, Roberts I, Bautista R, Caballero J, Coats T, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet. 2010;376:23–32. doi: 10.1016/S0140-6736(10)60835-5. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

