UBN1/2 of HIRA complex is responsible for recognition and deposition of H3.3 at cis-regulatory elements of genes in mouse ES cells
- PMID: 30285846
- PMCID: PMC6171237
- DOI: 10.1186/s12915-018-0573-9
UBN1/2 of HIRA complex is responsible for recognition and deposition of H3.3 at cis-regulatory elements of genes in mouse ES cells
Abstract
Background: H3.3 is an ancient and conserved H3 variant and plays essential roles in transcriptional regulation. HIRA complex, which is composed of HIRA, UBN1 or UBN2, and Cabin1, is a H3.3 specific chaperone complex. However, it still remains largely uncharacterized how HIRA complex specifically recognizes and deposits H3.3 to the chromatin, such as promoters and enhancers.
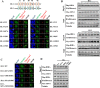
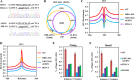
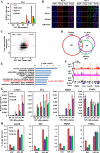
Results: In this study, we demonstrate that the UBN1 or UBN2 subunit is mainly responsible for specific recognition and direct binding of H3.3 by the HIRA complex. While the HIRA subunit can enhance the binding affinity of UBN1 toward H3.3, Cabin1 subunit cannot. We also demonstrate that both Ala87 and Gly90 residues of H3.3 are required and sufficient for the specific recognition and binding by UBN1. ChIP-seq studies reveal that two independent HIRA complexes (UBN1-HIRA and UBN2-HIRA) can cooperatively deposit H3.3 to cis-regulatory regions, including active promoters and active enhancers in mouse embryonic stem (mES) cells. Importantly, disruption of histone chaperone activities of UBN1 and UBN2 by FID/AAA mutation results in the defect of H3.3 deposition at promoters of developmental genes involved in neural differentiation, and subsequently causes the failure of activation of these genes during neural differentiation of mES cells.
Conclusion: Together, our results provide novel insights into the mechanism by which the HIRA complex specifically recognizes and deposits H3.3 at promoters and enhancers of developmental genes, which plays a critical role in neural differentiation of mES cells.
Keywords: Enhancer; HIRA complex; Histone variant H3.3; UBN1; UBN2.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



Similar articles
-
Human CABIN1 is a functional member of the human HIRA/UBN1/ASF1a histone H3.3 chaperone complex.Mol Cell Biol. 2011 Oct;31(19):4107-18. doi: 10.1128/MCB.05546-11. Epub 2011 Aug 1. Mol Cell Biol. 2011. PMID: 21807893 Free PMC article.
-
Placing the HIRA histone chaperone complex in the chromatin landscape.Cell Rep. 2013 Apr 25;3(4):1012-9. doi: 10.1016/j.celrep.2013.03.026. Epub 2013 Apr 18. Cell Rep. 2013. PMID: 23602572 Free PMC article.
-
Ubinuclein 2 is essential for mouse development and functions in X chromosome inactivation.PLoS Genet. 2025 Jun 2;21(6):e1011711. doi: 10.1371/journal.pgen.1011711. eCollection 2025 Jun. PLoS Genet. 2025. PMID: 40455860 Free PMC article.
-
A Molecular Prospective for HIRA Complex Assembly and H3.3-Specific Histone Chaperone Function.J Mol Biol. 2017 Jun 30;429(13):1924-1933. doi: 10.1016/j.jmb.2016.11.010. Epub 2016 Nov 19. J Mol Biol. 2017. PMID: 27871933 Free PMC article. Review.
-
Histone H3 dynamics in plant cell cycle and development.Cytogenet Genome Res. 2014;143(1-3):114-24. doi: 10.1159/000365264. Epub 2014 Jul 19. Cytogenet Genome Res. 2014. PMID: 25060842 Review.
Cited by
-
Dynamic Activity of Histone H3-Specific Chaperone Complexes in Oncogenesis.Front Oncol. 2022 Jan 11;11:806974. doi: 10.3389/fonc.2021.806974. eCollection 2021. Front Oncol. 2022. PMID: 35087762 Free PMC article. Review.
-
Histone variants: expanding the epigenetic potential of neurons one amino acid at a time.Trends Biochem Sci. 2025 Jun;50(6):532-543. doi: 10.1016/j.tibs.2025.03.015. Epub 2025 Apr 22. Trends Biochem Sci. 2025. PMID: 40268580 Review.
-
Independent and Complementary Functions of Caf1b and Hir1 for Chromatin Assembly in Tetrahymena thermophila.Cells. 2023 Dec 13;12(24):2828. doi: 10.3390/cells12242828. Cells. 2023. PMID: 38132148 Free PMC article.
-
Histone variant H2A.Z regulates nucleosome unwrapping and CTCF binding in mouse ES cells.Nucleic Acids Res. 2020 Jun 19;48(11):5939-5952. doi: 10.1093/nar/gkaa360. Nucleic Acids Res. 2020. PMID: 32392318 Free PMC article.
-
The ADAM9/UBN2/AKR1C3 axis promotes resistance to androgen-deprivation in prostate cancer.Am J Cancer Res. 2022 Jan 15;12(1):176-197. eCollection 2022. Am J Cancer Res. 2022. PMID: 35141012 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

