Adult Niemann-Pick disease type C in France: clinical phenotypes and long-term miglustat treatment effect
- PMID: 30285904
- PMCID: PMC6167825
- DOI: 10.1186/s13023-018-0913-4
Adult Niemann-Pick disease type C in France: clinical phenotypes and long-term miglustat treatment effect
Abstract
Background: Niemann-Pick disease type C (NP-C) is a neurodegenerative lysosomal lipid storage disease caused by autosomal recessive mutations in the NPC1 or NPC2 genes. The clinical presentation and evolution of NP-C and the effect of miglustat treatment are described in the largest cohort of patients with adolescent/adult-onset NP-C studied to date.
Methods: Observational study based on clinical chart data from adult patients with NP-C (> 18 year old) diagnosed in France between 1990 and 2015. Retrospective data from patients at diagnosis, onset of miglustat therapy (if applicable), and last follow up were analysed.
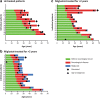
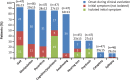
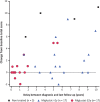
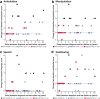
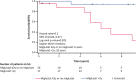
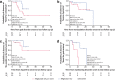
Results: In France, patients with an adolescent-adult neurological form constituted approximately 25% of all NP-C cases diagnosed during the study period. Forty-seven patients (46 with NP-C1 and one with NP-C2; 53% female) were included. Mean ± SD (range) ages at neurological onset and diagnosis were 23.9 ± 12.5 (8-56) years and 34 ± 13.5 (15-65) years, respectively. At presentation, patients mainly had 1) impaired gait due to cerebellar ataxia and/or dystonia, 2) and/or cognitive/behavioural manifestations, 3) and/or psychotic signs. Initially, almost half of patients had only one of the above three neuro-psychiatric manifestations. Vertical supranuclear gaze palsy, usually occurring without patient complaint, was only detected on careful clinical examination and was recorded in most patients (93%) at the time of diagnosis, several years after neurological onset. Thirty-seven patients (79%) received miglustat, among whom seventeen (46%) continued beyond 2 years (at last follow up) to a maximum of 9.8 years. Eight patients (22%) discontinued treatment early due to side effects (n = 3) or perceived lack of efficacy (n = 5).Miglustat treatment duration correlated significantly with reduced neurological worsening (p < 0.001). Treatment for≥2 years was associated with improved patient survival (p = 0.029). Good responses to miglustat were associated with less severe neurological disability at the start of miglustat treatment (p = 0.02).
Conclusion: The proportion of adolescent/adult-onset NP-C cases diagnosed in France increased 2.5-fold since 2009 compared with the 2000-2008 period due to improved awareness. Adolescent/adult-onset NP-C frequently presented initially with a non-specific isolated neuro-psychiatric manifestation (motor, cognitive or psychotic). Patients with less severe neurological disability responded better to miglustat therapy.
Keywords: Adult-onset; Efficacy; Epidemiology; France; Miglustat; Niemann-pick disease type C; Safety.
Conflict of interest statement
Ethics approval and consent to participate
Local ethics committee approval of the study was obtained from the CPP – Ile-de-France.
Consent for publication
Not applicable.
Competing interests
YN received speech honoraria from Actelion and Orphan Europe; and received travel funding from Actelion, Shire and Genzyme. PL has received presentation honoraria from Actelion Pharmaceuticals France. CG received from Actelion honorarium for participation in an advisory board and funding for inscriptions and travels for congresses. MA declares honoraria from Actelion, Abbvie and Teva. ER received research support from Merz-Pharma, Orkyn, Aguettant, IP Santé, Ultragenix, and UCB pharma; served on scientific advisory boards for Orkyn, Ultragenix, Retrophin and Merz-Pharma; received speech honoraria from Orkyn, Aguettant, Merz-Pharma and Ultragenix; and received travel funding from the Elivie, the Dystonia Coalition, the Dystonia Medical Research Foundation, the Movement Disorders Society, the European Academy of Neurology. MTV has received honoraria and travel reimbursement from Actelion Pharmaceuticals Ltd., Sucampo Pharmaceuticals Inc. and Mallinckrodt Pharmaceuticals as a member of advisory committees and/or guest speaker, travel reimbursement from Vtesse, and honoraria from Shire as a member of a data and safety monitoring board. BH has received travel expenses from, and attended meetings funded and organized by Actelion Pharmaceuticals Ltd., Biomarin, Genzyme Corporation and Shire HGT, and has received presentation honoraria from Actelion Pharmaceuticals Ltd. ALHM, XA, CT, PC, AD, CL, LM, CT, BA, VD, TDdG, and FL have no conflicts of interest to declare.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Miglustat therapy in the French cohort of paediatric patients with Niemann-Pick disease type C.Orphanet J Rare Dis. 2012 Jun 7;7:36. doi: 10.1186/1750-1172-7-36. Orphanet J Rare Dis. 2012. PMID: 22676771 Free PMC article. Clinical Trial.
-
Disease characteristics, prognosis and miglustat treatment effects on disease progression in patients with Niemann-Pick disease Type C: an international, multicenter, retrospective chart review.Orphanet J Rare Dis. 2019 Jan 8;14(1):32. doi: 10.1186/s13023-019-0996-6. Orphanet J Rare Dis. 2019. PMID: 30732631 Free PMC article.
-
Stable or improved neurological manifestations during miglustat therapy in patients from the international disease registry for Niemann-Pick disease type C: an observational cohort study.Orphanet J Rare Dis. 2015 May 28;10:65. doi: 10.1186/s13023-015-0284-z. Orphanet J Rare Dis. 2015. PMID: 26017010 Free PMC article.
-
[Adult onset Niemann-Pick type C disease and psychosis: literature review].Encephale. 2013 Oct;39(5):315-9. doi: 10.1016/j.encep.2013.04.013. Epub 2013 Aug 5. Encephale. 2013. PMID: 23928063 Review. French.
-
Miglustat in Niemann-Pick disease type C patients: a review.Orphanet J Rare Dis. 2018 Aug 15;13(1):140. doi: 10.1186/s13023-018-0844-0. Orphanet J Rare Dis. 2018. PMID: 30111334 Free PMC article. Review.
Cited by
-
Challenges in the Definitive Diagnosis of Niemann-Pick Type C-Leaky Variants and Alternative Transcripts.Genes (Basel). 2023 Oct 25;14(11):1990. doi: 10.3390/genes14111990. Genes (Basel). 2023. PMID: 38002933 Free PMC article. Review.
-
The Classification of Autosomal Recessive Cerebellar Ataxias: a Consensus Statement from the Society for Research on the Cerebellum and Ataxias Task Force.Cerebellum. 2019 Dec;18(6):1098-1125. doi: 10.1007/s12311-019-01052-2. Cerebellum. 2019. PMID: 31267374 Free PMC article. Review.
-
Transcript, protein, metabolite and cellular studies in skin fibroblasts demonstrate variable pathogenic impacts of NPC1 mutations.Orphanet J Rare Dis. 2020 Apr 5;15(1):85. doi: 10.1186/s13023-020-01360-5. Orphanet J Rare Dis. 2020. PMID: 32248828 Free PMC article.
-
Understanding and Treating Niemann-Pick Type C Disease: Models Matter.Int J Mol Sci. 2020 Nov 26;21(23):8979. doi: 10.3390/ijms21238979. Int J Mol Sci. 2020. PMID: 33256121 Free PMC article. Review.
-
Neurofilament light chain in cerebrospinal fluid as a novel biomarker in evaluating both clinical severity and therapeutic response in Niemann-Pick disease type C1.Genet Med. 2023 Mar;25(3):100349. doi: 10.1016/j.gim.2022.11.017. Epub 2022 Dec 5. Genet Med. 2023. PMID: 36470574 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

