High tension in sarcomeres hinders myocardial relaxation: A computational study
- PMID: 30286135
- PMCID: PMC6171862
- DOI: 10.1371/journal.pone.0204642
High tension in sarcomeres hinders myocardial relaxation: A computational study
Abstract
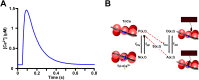
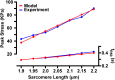
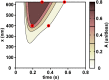
Experiments have shown that the relaxation phase of cardiac sarcomeres during an isometric twitch is prolonged in muscles that reached a higher peak tension. However, the mechanism is not completely understood. We hypothesize that the binding of calcium to troponin is enhanced by the tension in the thin filament, thus contributing to the prolongation of contraction upon higher peak tension generation. To test this hypothesis, we developed a computational model of sarcomere mechanics that incorporates tension-dependence of calcium binding. The model was used to simulate isometric twitch experiments with time dependency in the form of a two-state cross-bridge cycle model and a transient intracellular calcium concentration. In the simulations, peak isometric twitch tension appeared to increase linearly by 51.1 KPa with sarcomere length from 1.9 μm to 2.2 μm. Experiments showed an increase of 47.3 KPa over the same range of sarcomere lengths. The duration of the twitch also increased with both sarcomere length and peak intracellular calcium concentration, likely to be induced by the inherently coupled increase of the peak tension in the thin filament. In the model simulations, the time to 50% relaxation (tR50) increased over the range of sarcomere lengths from 1.9 μm to 2.2 μm by 0.11s, comparable to the increased duration of 0.12s shown in experiments. Model simulated tR50 increased by 0.12s over the range of peak intracellular calcium concentrations from 0.87 μM to 1.45 μM. Our simulation results suggest that the prolongation of contraction at higher tension is a result of the tighter binding of Ca2+ to troponin in areas under higher tension, thus delaying the deactivation of the troponin.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Kentish JC, ter Keurs HE, Ricciardi L, Bucx JJ, Noble MI. Comparison between the sarcomere length-force relations of intact and skinned trabeculae from rat right ventricle. Influence of calcium concentrations on these relations. Circulation Research. 1986;58(6):755–68. 10.1161/01.res.58.6.755 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

