Tumorigenicity-associated characteristics of human iPS cell lines
- PMID: 30286143
- PMCID: PMC6171902
- DOI: 10.1371/journal.pone.0205022
Tumorigenicity-associated characteristics of human iPS cell lines
Abstract
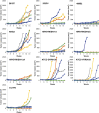
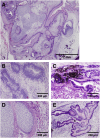
Human induced pluripotent stem cells (hiPSCs) represent promising raw materials of human cell-based therapeutic products (hCTPs). As undifferentiated hiPSCs exhibit intrinsic tumorigenicity properties that enable them to form teratomas, hCTPs containing residual undifferentiated hiPSCs may cause tumor formation following transplantation. We first established quantitative and sensitive tumorigenicity testing of hiPSCs dissociated into single cells using NOD/Shi-scid IL2Rγnull (NOG) mice by inhibiting apoptosis of hiPSCs with a Rho kinase inhibitor. To examine different features in tumorigenicity of various hiPSCs, 10 commonly available hiPSC lines were subjected to in vivo tumorigenicity testing. Transplanted hiPSC lines showed remarkable variation in tumor incidence, formation latency, and volumes. Most of the tumors formed were classified as immature teratomas. However, no signs of malignancies, such as carcinoma and sarcoma, were recognized in the tumors. Characteristics associated tumorigenicity of hiPSCs were investigated with microarray analysis, karyotype analysis, and whole exome sequencing. Gene expression profiling and pathway analysis supported different features of hiPSC lines in tumorigenicity. hiPSC lines showed chromosomal abnormalities in some lines and 61-77 variants of cancer-related genes carrying effective nonsynonymous mutations, which were confirmed in the COSMIC databases. In this study, the chromosomal abnormalities and cancer-related gene mutations observed in hiPSC lines did not lead to the malignancy of tumors derived from hiPSCs. Our results suggest that the potential tumorigenicity risk of hCTPs containing residual undifferentiated hiPSCs is dependent on not only amounts of undifferentiated hiPSCs but also features of the cell lines used as raw materials, a finding that should be considered from the perspective of quality of hCTPs used.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



Similar articles
-
Aurora-A overexpression is linked to development of aggressive teratomas derived from human iPS cells.Oncol Rep. 2018 Apr;39(4):1725-1730. doi: 10.3892/or.2018.6239. Epub 2018 Jan 31. Oncol Rep. 2018. PMID: 29393405 Free PMC article.
-
Whole-Genome DNA Methylation Analyses Revealed Epigenetic Instability in Tumorigenic Human iPS Cell-Derived Neural Stem/Progenitor Cells.Stem Cells. 2017 May;35(5):1316-1327. doi: 10.1002/stem.2581. Epub 2017 Feb 23. Stem Cells. 2017. PMID: 28142229
-
Tumorigenic risk of human induced pluripotent stem cell explants cultured on mouse SNL76/7 feeder cells.Biochem Biophys Res Commun. 2014 Oct 24;453(3):668-73. doi: 10.1016/j.bbrc.2014.10.009. Epub 2014 Oct 8. Biochem Biophys Res Commun. 2014. PMID: 25305485
-
[In vitro tumorigenicity tests for process control of health care products derived from human induced pluripotent stem cells].Yakugaku Zasshi. 2013;133(12):1381-8. doi: 10.1248/yakushi.13-00232-3. Yakugaku Zasshi. 2013. PMID: 24292187 Review. Japanese.
-
The tumorigenicity of diploid and aneuploid human pluripotent stem cells.Cell Cycle. 2009 Dec;8(23):3822-30. doi: 10.4161/cc.8.23.10067. Epub 2009 Dec 14. Cell Cycle. 2009. PMID: 19887907 Review.
Cited by
-
The potential and limitations of induced pluripotent stem cells to achieve wound healing.Stem Cell Res Ther. 2019 Mar 12;10(1):87. doi: 10.1186/s13287-019-1185-1. Stem Cell Res Ther. 2019. PMID: 30867069 Free PMC article. Review.
-
Personalized Stem Cell-Based Regeneration in Spinal Cord Injury Care.Int J Mol Sci. 2025 Apr 19;26(8):3874. doi: 10.3390/ijms26083874. Int J Mol Sci. 2025. PMID: 40332538 Free PMC article. Review.
-
Predicting differentiation potential of human pluripotent stem cells: Possibilities and challenges.World J Stem Cells. 2019 Jul 26;11(7):375-382. doi: 10.4252/wjsc.v11.i7.375. World J Stem Cells. 2019. PMID: 31396366 Free PMC article.
-
Elimination of tumorigenic pluripotent stem cells from their differentiated cell therapy products: An important step toward ensuring safe cell therapy.Stem Cell Reports. 2025 Jul 8;20(7):102543. doi: 10.1016/j.stemcr.2025.102543. Epub 2025 Jun 19. Stem Cell Reports. 2025. PMID: 40541178 Free PMC article. Review.
-
Development of a novel gene expression panel for the characterization of MSCs for increased biological safety.J Appl Genet. 2025 Sep;66(3):623-636. doi: 10.1007/s13353-024-00917-5. Epub 2024 Dec 2. J Appl Genet. 2025. PMID: 39621192 Free PMC article.
References
-
- WHO Expert Committee on Biological Standardization: Sixty-first Report. WHO Technical Report Series, No. 978 Annex 3. Recommendations for the evaluation of animal cell cultures as substrates for the manufacture of biological medicinal products and for the characterization of cell banks: World Health Organization; 2013. 79–187 p.
-
- Kusakawa S, Machida K, Yasuda S, Takada N, Kuroda T, Sawada R, et al. Characterization of in vivo tumorigenicity tests using severe immunodeficient NOD/Shi-scid IL2Rgnull mice for detection of tumorigenic cellular impurities in human cell-processed therapeutic products. Regen Ther. 2015;1:30–7. - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

