Germ cell desquamation-based testis regression in a seasonal breeder, the Egyptian long-eared hedgehog, Hemiechinus auritus
- PMID: 30286149
- PMCID: PMC6171879
- DOI: 10.1371/journal.pone.0204851
Germ cell desquamation-based testis regression in a seasonal breeder, the Egyptian long-eared hedgehog, Hemiechinus auritus
Abstract
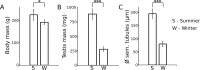
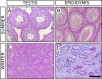
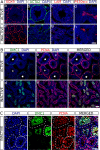
Testes of seasonally breeding species experience a severe functional regression before the non-breeding period, which implies a substantial mass reduction due to massive germ-cell depletion. Two alternative mechanisms of seasonal germ-cell depletion have been described in mammals, apoptosis and desquamation (sloughing), but their prevalence has not been determined yet due to reduced number of species studied. We performed a morphological, hormonal, and molecular study of the mechanism of seasonal testicular regression in males of the Egyptian long eared-hedgehog (Hemiechinus auritus). Our results show that live, non-apoptotic, germ cells are massively depleted by desquamation during the testis regression process. This is concomitant with both decreased levels of serum testosterone and irregular distribution of the cell-adhesion molecules in the seminiferous epithelium. The inactive testes maintain some meiotic activity as meiosis onset is not halted and spermatocytes die by apoptosis at the pachytene stage. Our data support the notion that apoptosis is not the major testis regression effector in mammals. Instead, desquamation appears to be a common mechanism in this class.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Neill JD. Knobil and Neill’s physiology of reproduction: Academic Press; 2006. 3296 p.
-
- Dadhich RK, Real FM, Zurita F, Barrionuevo FJ, Burgos M, Jimenez R. Role of apoptosis and cell proliferation in the testicular dynamics of seasonal breeding mammals: a study in the Iberian mole, Talpa occidentalis. Biology of reproduction. 2010;83:83–91. 10.1095/biolreprod.109.080135 . - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

