Functional analysis of a triplet deletion in the gene encoding the sodium glucose transporter 3, a potential risk factor for ADHD
- PMID: 30286162
- PMCID: PMC6171906
- DOI: 10.1371/journal.pone.0205109
Functional analysis of a triplet deletion in the gene encoding the sodium glucose transporter 3, a potential risk factor for ADHD
Abstract
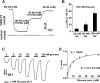
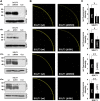
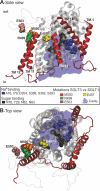
Sodium-glucose transporters (SGLT) belong to the solute carrier 5 family, which is characterized by sodium dependent transport of sugars and other solutes. In contrast, the human SGLT3 (hSGLT3) isoform, encoded by SLC5A4, acts as a glucose sensor that does not transport sugar but induces membrane depolarization by Na+ currents upon ligand binding. Whole-exome sequencing (WES) of several extended pedigrees with high density of attention-deficit/hyperactivity disorder (ADHD) identified a triplet ATG deletion in SLC5A4 leading to a single amino acid loss (ΔM500) in the hSGLT3 protein imperfectly co-segregating with the clinical phenotype of ADHD. Since mutations in homologous domains of hSGLT1 and hSGLT2 were found to affect intestinal and renal function, respectively, we analyzed the functional properties of hSGLT3[wt] and [ΔM500] by voltage clamp and current clamp recordings from cRNA-injected Xenopus laevis oocytes. The cation conductance of hSGLT3[wt] was activated by application of glucose or the specific agonist 1-desoxynojirimycin (DNJ) as revealed by inward currents in the voltage clamp configuration and cell depolarization in the current clamp mode. Almost no currents and changes in membrane potential were observed when glucose or DNJ were applied to hSGLT3[ΔM500]-injected oocytes, demonstrating a loss of function by this amino acid deletion in hSGLT3. To monitor membrane targeting of wt and mutant hSGLT3, fusion constructs with YFP were generated, heterologously expressed in Xenopus laevis oocytes and analyzed for membrane fluorescence by confocal microscopy. In comparison to hSGLT3[wt] the fluorescent signal of mutant [ΔM500] was reduced by 43% indicating that the mutant phenotype might mainly result from inaccurate membrane targeting. As revealed by homology modeling, residue M500 is located in TM11 suggesting that in addition to the core structure (TM1-TM10) of the transporter, the surrounding TMs are equally crucial for transport/sensor function. In conclusion, our findings indicate that the deletion [ΔM500] in hSGLT3 inhibits membrane targeting and thus largely disrupts glucose-induced sodium conductance, which may, in interaction with other ADHD risk-related gene variants, influence the risk for ADHD in deletion carriers.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Wright EM. Glucose transport families SLC5 and SlC50. Mol Aspects Med. 2013;34: 182–196. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

