Desmoplakin interacts with the coil 1 of different types of intermediate filament proteins and displays high affinity for assembled intermediate filaments
- PMID: 30286183
- PMCID: PMC6171917
- DOI: 10.1371/journal.pone.0205038
Desmoplakin interacts with the coil 1 of different types of intermediate filament proteins and displays high affinity for assembled intermediate filaments
Abstract
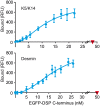
The interaction of intermediate filaments (IFs) with the cell-cell adhesion complexes desmosomes is crucial for cytoskeletal organization and cell resilience in the epidermis and heart. The intracellular desmosomal protein desmoplakin anchors IFs to the cell adhesion complexes predominantly via its four last carboxy-terminal domains (C-terminus). However, it remains unclear why the C-terminus of desmoplakin interacts with different IF types or if there are different binding affinities for each type of IFs that may influence the stability of cell-specific adhesion complexes. By yeast three-hybrid and fluorescence binding assays, we found that the coiled-coil 1 of the conserved central rod domain of the heterodimeric cytokeratins (Ks) 5 and 14 (K5/K14) was required for their interaction with the C-terminus of desmoplakin, while their unique amino head- and C-tail domains were dispensable. Similar findings were obtained in vitro with K1/K10, and the type III IF proteins desmin and vimentin. Binding assays testing the C-terminus of desmoplakin with assembled K5/K14 and desmin IFs yielded an apparent affinity in the nM range. Our findings reveal that the same conserved domain of IF proteins binds to the C-terminus of desmoplakin, which may help explain the previously reported broad binding IF-specificity to desmoplakin. Our data suggest that desmoplakin high-affinity binding to diverse IF proteins ensures robust linkages of IF cytoskeleton and desmosomes that maintain the structural integrity of cellular adhesion complexes. In summary, our results give new insights into the molecular basis of the IF-desmosome association.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Gallicano GI, Bauer C, Fuchs E. Rescuing desmoplakin function in extra-embryonic ectoderm reveals the importance of this protein in embryonic heart, neuroepithelium, skin and vasculature. Development. 2001;128(6):929–41. Epub 2001/02/27. . - PubMed
-
- Franke WW, Borrmann CM, Grund C, Pieperhoff S. The area composita of adhering junctions connecting heart muscle cells of vertebrates. I. Molecular definition in intercalated disks of cardiomyocytes by immunoelectron microscopy of desmosomal proteins. Eur J Cell Biol. 2006;85(2):69–82. Epub 2006/01/13. 10.1016/j.ejcb.2005.11.003 . - DOI - PubMed
-
- Kowalczyk AP, Hatzfeld M, Bornslaeger EA, Kopp DS, Borgwardt JE, Corcoran CM, et al. The head domain of plakophilin-1 binds to desmoplakin and enhances its recruitment to desmosomes. Implications for cutaneous disease. J Biol Chem. 1999;274(26):18145–8. Epub 1999/06/22. . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

