Association Between Aspirin Use and Risk of Hepatocellular Carcinoma
- PMID: 30286235
- PMCID: PMC6440745
- DOI: 10.1001/jamaoncol.2018.4154
Association Between Aspirin Use and Risk of Hepatocellular Carcinoma
Erratum in
-
Incomplete Conflict of Interest Disclosures.JAMA Oncol. 2019 Apr 1;5(4):579. doi: 10.1001/jamaoncol.2019.0286. JAMA Oncol. 2019. PMID: 30844032 Free PMC article. No abstract available.
Abstract
Importance: Prospective data on the risk of hepatocellular carcinoma (HCC) according to dose and duration of aspirin therapy are limited.
Objective: To examine the potential benefits of aspirin use for primary HCC prevention at a range of doses and durations of use within 2 prospective, nationwide populations.
Design, setting, and participants: Pooled analysis of 2 prospective US cohort studies: the Nurses' Health Study and the Health Professionals Follow-up Study. Data were accessed from November 1, 2017, through March 7, 2018. A total of 133 371 health care professionals who reported data on aspirin use, frequency, dosage, and duration of use biennially since 1980 in women and 1986 in men were included. Individuals with a cancer diagnosis at baseline (except nonmelanoma skin cancer) were excluded.
Main outcomes and measures: Cox proportional hazards regression models were used to calculate multivariable adjusted hazard ratios (HRs) and 95% CIs for HCC.
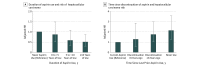
Results: Of the 133 371 participants, 87 507 were women and 45 864 were men; in 1996, the median time of follow-up, the mean (SD) age was 62 (8) years for women and 64 (8) years for men. Over more than 26 years of follow-up encompassing 4 232 188 person-years, 108 incident HCC cases (65 women, 43 men) were documented. Compared with nonregular use, regular aspirin use (≥2 standard-dose [325-mg] tablets per week) was associated with reduced HCC risk (adjusted HR, 0.51; 95% CI, 0.34-0.77). This benefit appeared to be dose related: compared with nonuse, the multivariable-adjusted HR for HCC was 0.87 (95% CI, 0.51-1.48) for up to 1.5 standard-dose tablets per week, 0.51 (95% CI, 0.30-0.86) for more than 1.5 to 5 tablets per week, and 0.49 (95% CI, 0.28-0.96) for more than 5 tablets per week (P for trend = .006). Significantly lower HCC risk was observed with increasing duration (P for trend = .03); this decrease was apparent with use of 1.5 or more standard-dose aspirin tablets per week for 5 or more years (adjusted HR, 0.41; 95% CI, 0.21-0.77). In contrast, use of nonaspirin nonsteroidal anti-inflammatory drugs was not significantly associated with HCC risk (adjusted HR, 1.09; 95% CI, 0.78-1.51).
Conclusions and relevance: This study suggests that regular, long-term aspirin use is associated with a dose-dependent reduction in HCC risk, which is apparent after 5 or more years of use. Similar associations were not found with nonaspirin NSAIDs. Further research appears to be needed to clarify whether aspirin use represents a feasible strategy for primary prevention against HCC.
Conflict of interest statement
Figures
Comment in
-
Aspirin and Chemoprevention-Have We Arrived?JAMA Oncol. 2018 Dec 1;4(12):1668-1669. doi: 10.1001/jamaoncol.2018.4138. JAMA Oncol. 2018. PMID: 30286216 No abstract available.
-
Omitted Disclosures of Potential Conflicts of Interest in Articles Published in JAMA Oncology.JAMA Oncol. 2019 Apr 1;5(4):578-579. doi: 10.1001/jamaoncol.2019.0235. JAMA Oncol. 2019. PMID: 30844040 No abstract available.
-
Reduced Hepatocellular Carcinoma Risk vs Bleeding Risk Associated With Aspirin.JAMA Oncol. 2019 Jun 1;5(6):911. doi: 10.1001/jamaoncol.2019.0614. JAMA Oncol. 2019. PMID: 31046061 No abstract available.
-
Aspirin Use and the Risk of Cancer.JAMA Oncol. 2019 Jun 1;5(6):912-913. doi: 10.1001/jamaoncol.2019.0611. JAMA Oncol. 2019. PMID: 31046062 No abstract available.
-
Reduced Hepatocellular Carcinoma Risk vs Bleeding Risk Associated With Aspirin-In Reply.JAMA Oncol. 2019 Jun 1;5(6):911-912. doi: 10.1001/jamaoncol.2019.0630. JAMA Oncol. 2019. PMID: 31046066 No abstract available.
-
Aspirin Use and the Risk of Cancer-In Reply.JAMA Oncol. 2019 Jun 1;5(6):913. doi: 10.1001/jamaoncol.2019.0633. JAMA Oncol. 2019. PMID: 31046070 No abstract available.
-
Dose and Duration of Aspirin Use to Reduce Incident Hepatocellular Carcinoma.Hepatology. 2019 Dec;70(6):2216-2217. doi: 10.1002/hep.30813. Epub 2019 Aug 7. Hepatology. 2019. PMID: 31206214 Free PMC article. No abstract available.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

