Donor APOL1 high-risk genotypes are associated with increased risk and inferior prognosis of de novo collapsing glomerulopathy in renal allografts
- PMID: 30287079
- PMCID: PMC6251748
- DOI: 10.1016/j.kint.2018.06.024
Donor APOL1 high-risk genotypes are associated with increased risk and inferior prognosis of de novo collapsing glomerulopathy in renal allografts
Abstract
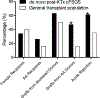
Collapsing focal segmental glomerulosclerosis (cFSGS) in the native kidney is associated with heavy proteinuria and accelerated renal failure. However, cFSGS in the renal allograft is less well characterized. Here we report clinico-pathologic features and APOL1 donor risk genotypes in 38 patients with de novo post-kidney transplant cFSGS. Recipients were 34% female and 26% African American. Concurrent viral infections and acute vaso-occlusion (including thrombotic microangiopathy, cortical necrosis, atheroembolization, and cardiac arrest with contralateral graft thrombosis) were present in 13% and 29% of recipients, respectively. Notably, 61% of patients had concurrent acute rejection and 47% received grafts from African American donors, of which 53% carried APOL1 high-risk genotypes. These frequencies of acute rejection and grafts from African American donors were significantly higher than in our general transplant population (35% and 16%, respectively). Patients had a median serum creatinine of 5.4 mg/dl, urine protein/creatinine 3.5 g/g, and 18% had nephrotic syndrome. Graft failure occurred in 63% of patients at an average of eighteen months post-index biopsy. By univariate analysis, donor APOL1 high-risk genotypes, post-transplant time, nephrotic syndrome, and chronic histologic changes were associated with inferior graft survival while acute vaso-occlusion was associated with superior graft survival. Donor APOL1 high-risk genotypes independently predicted poor outcome. Compared to native kidney cFSGS, post-transplant cFSGS had more acute vaso-occlusion but less proteinuria. Thus, de novo cFSGS is associated with variable proteinuria and poor prognosis with potential predisposing factors of African American donor, acute rejection, viral infection and acute vaso-occlusion. Additionally, donor APOL1 high-risk genotypes are associated with higher incidence and worse graft survival.
Keywords: APOL1 genotypes; African Americans; acute rejection; acute vasoocclusion; collapsing glomerulopathy; kidney transplantation.
Copyright © 2018 International Society of Nephrology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures


Comment in
-
Expanding the spectrum of APOL1-related renal disease: de novo collapsing glomerulopathy following kidney transplant.Kidney Int. 2018 Dec;94(6):1048-1050. doi: 10.1016/j.kint.2018.09.006. Kidney Int. 2018. PMID: 30466562 Free PMC article.
Similar articles
-
Donor's APOL1 Risk Genotype and "Second Hits" Associated With De Novo Collapsing Glomerulopathy in Deceased Donor Kidney Transplant Recipients: A Report of 5 Cases.Am J Kidney Dis. 2019 Jan;73(1):134-139. doi: 10.1053/j.ajkd.2018.05.008. Epub 2018 Jul 25. Am J Kidney Dis. 2019. PMID: 30054024 Free PMC article.
-
Collapsing and non-collapsing focal segmental glomerulosclerosis in kidney transplants.Nephrol Dial Transplant. 2006 Sep;21(9):2607-14. doi: 10.1093/ndt/gfl225. Epub 2006 May 16. Nephrol Dial Transplant. 2006. PMID: 16705026
-
APOL1 Genotype and Kidney Transplantation Outcomes From Deceased African American Donors.Transplantation. 2016 Jan;100(1):194-202. doi: 10.1097/TP.0000000000000969. Transplantation. 2016. PMID: 26566060 Free PMC article.
-
Transplantation With APOL1 Risk Variant Kidney May Be Associated With Lifetime Risk for Recurrence of Focal Segmental Glomerulosclerosis: A Case Report and Review of Literature.Transplant Proc. 2019 Nov;51(9):3077-3079. doi: 10.1016/j.transproceed.2019.04.056. Epub 2019 Jul 16. Transplant Proc. 2019. PMID: 31324484 Review.
-
Transplant capillaropathy and transplant glomerulopathy: ultrastructural markers of chronic renal allograft rejection.Nephrol Dial Transplant. 2003 Apr;18(4):655-60. doi: 10.1093/ndt/gfg139. Nephrol Dial Transplant. 2003. PMID: 12637631 Review. No abstract available.
Cited by
-
CMV-associated collapsing focal segmental glomerulosclerosis after kidney transplant in a pediatric patient.Pediatr Transplant. 2023 Aug;27(5):e14535. doi: 10.1111/petr.14535. Epub 2023 May 1. Pediatr Transplant. 2023. PMID: 37128132 Free PMC article.
-
Granulomatous Tubulointerstitial Nephritis as a Rare Cause of Allograft Failure: A Case Report.Cureus. 2024 Dec 24;16(12):e76353. doi: 10.7759/cureus.76353. eCollection 2024 Dec. Cureus. 2024. PMID: 39866980 Free PMC article.
-
Variant APOL1 protein in plasma associates with larger particles in humans and mouse models of kidney injury.PLoS One. 2022 Oct 24;17(10):e0276649. doi: 10.1371/journal.pone.0276649. eCollection 2022. PLoS One. 2022. PMID: 36279295 Free PMC article.
-
Post-transplant glomerular diseases: update on pathophysiology, risk factors and management strategies.Clin Kidney J. 2024 Oct 24;17(12):sfae320. doi: 10.1093/ckj/sfae320. eCollection 2024 Dec. Clin Kidney J. 2024. PMID: 39664990 Free PMC article. Review.
-
Monogenic Kidney Diseases in Kidney Transplantation.Kidney Int Rep. 2023 Dec 13;9(3):549-568. doi: 10.1016/j.ekir.2023.12.003. eCollection 2024 Mar. Kidney Int Rep. 2023. PMID: 38481491 Free PMC article. Review.
References
-
- D’Agati VD, Fogo AB, Bruijn JA, et al. Pathologic classification of focal segmental glomerulosclerosis: a working proposal. Am J Kidney Dis 2004; 43: 368–382. - PubMed
-
- Laurinavicius A, Hurwitz S, Rennke HG. Collapsing glomerulopathy in HIV and non-HIV patients: a clinicopathological and follow-up study. Kidney Int 1999; 56: 2203–2213. - PubMed
-
- Albaqumi M, Soos TJ, Barisoni L, et al. Collapsing glomerulopathy. Journal of the American Society of Nephrology : JASN 2006; 17: 2854–2863. - PubMed
-
- Detwiler RK, Falk RJ, Hogan SL, et al. Collapsing glomerulopathy: a clinically and pathologically distinct variant of focal segmental glomerulosclerosis. Kidney Int 1994; 45: 1416–1424. - PubMed
-
- Valeri A, Barisoni L, Appel GB, et al. Idiopathic collapsing focal segmental glomerulosclerosis: a clinicopathologic study. Kidney Int 1996; 50: 1734–1746. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

