Native-state imaging of calcifying and noncalcifying microalgae reveals similarities in their calcium storage organelles
- PMID: 30287487
- PMCID: PMC6205483
- DOI: 10.1073/pnas.1804139115
Native-state imaging of calcifying and noncalcifying microalgae reveals similarities in their calcium storage organelles
Abstract
Calcium storage organelles are common to all eukaryotic organisms and play a pivotal role in calcium signaling and cellular calcium homeostasis. In most organelles, the intraorganellar calcium concentrations rarely exceed micromolar levels. Acidic organelles called acidocalcisomes, which concentrate calcium into dense phases together with polyphosphates, are an exception. These organelles have been identified in diverse organisms, but, to date, only in cells that do not form calcium biominerals. Recently, a compartment storing molar levels of calcium together with phosphorous was discovered in an intracellularly calcifying alga, the coccolithophore Emiliania huxleyi, raising a possible connection between calcium storage organelles and calcite biomineralization. Here we used cryoimaging and cryospectroscopy techniques to investigate the anatomy and chemical composition of calcium storage organelles in their native state and at nanometer-scale resolution. We show that the dense calcium phase inside the calcium storage compartment of the calcifying coccolithophore Pleurochrysis carterae and the calcium phase stored in acidocalcisomes of the noncalcifying alga Chlamydomonas reinhardtii have common features. Our observations suggest that this strategy for concentrating calcium is a widespread trait and has been adapted for coccolith formation. The link we describe between acidocalcisomal calcium storage and calcium storage in coccolithophores implies that our physiological and molecular genetic understanding of acidocalcisomes could have relevance to the calcium pathway underlying coccolithophore calcification, offering a fresh entry point for mechanistic investigations on the adaptability of this process to changing oceanic conditions.
Keywords: acidocalcisome; biomineralization; coccolithophore; cryo–X-ray tomography; intracellular calcium store.
Copyright © 2018 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


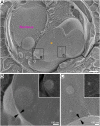
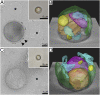
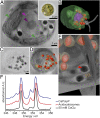
Similar articles
-
Exploring Intracellular Ion Pools in Coccolithophores Using Live-Cell Imaging.Adv Biol (Weinh). 2021 Jun;5(6):e2000296. doi: 10.1002/adbi.202000296. Epub 2021 Apr 14. Adv Biol (Weinh). 2021. PMID: 33852773
-
Regulation of CaCO(3) formation in coccolithophores.Comp Biochem Physiol B Biochem Mol Biol. 2003 Dec;136(4):743-54. doi: 10.1016/s1096-4959(03)00180-5. Comp Biochem Physiol B Biochem Mol Biol. 2003. PMID: 14662299 Review.
-
Biomineralization pathways in calcifying dinoflagellates: Uptake, storage in MgCaP-rich bodies and formation of the shell.Acta Biomater. 2020 Jan 15;102:427-439. doi: 10.1016/j.actbio.2019.11.042. Epub 2019 Nov 27. Acta Biomater. 2020. PMID: 31785382
-
Formation and mosaicity of coccolith segment calcite of the marine algae Emiliania huxleyi.J Phycol. 2018 Feb;54(1):85-104. doi: 10.1111/jpy.12604. Epub 2017 Nov 22. J Phycol. 2018. PMID: 29092105
-
Biomineralization in coccolithophores.Gravit Space Biol Bull. 1999 May;12(2):5-14. Gravit Space Biol Bull. 1999. PMID: 11541783 Review.
Cited by
-
Soft X-ray tomography analysis of mitochondria dynamics in Saccharomyces cerevisiae.Biol Direct. 2024 Nov 29;19(1):126. doi: 10.1186/s13062-024-00570-2. Biol Direct. 2024. PMID: 39614383 Free PMC article.
-
The cellular landscape by cryo soft X-ray tomography.Biophys Rev. 2019 Jun 8;11(4):611-619. doi: 10.1007/s12551-019-00567-6. Epub 2019 Jul 4. Biophys Rev. 2019. PMID: 31273607 Free PMC article. Review.
-
A joint proteomic and genomic investigation provides insights into the mechanism of calcification in coccolithophores.Nat Commun. 2023 Jun 23;14(1):3749. doi: 10.1038/s41467-023-39336-1. Nat Commun. 2023. PMID: 37353496 Free PMC article.
-
Intracellular nanoscale architecture as a master regulator of calcium carbonate crystallization in marine microalgae.Proc Natl Acad Sci U S A. 2021 Nov 16;118(46):e2025670118. doi: 10.1073/pnas.2025670118. Proc Natl Acad Sci U S A. 2021. PMID: 34772804 Free PMC article.
-
Calcite as a Precursor of Hydroxyapatite in the Early Biomineralization of Differentiating Human Bone-Marrow Mesenchymal Stem Cells.Int J Mol Sci. 2021 May 6;22(9):4939. doi: 10.3390/ijms22094939. Int J Mol Sci. 2021. PMID: 34066542 Free PMC article.
References
-
- Campbell AK. Intracellular Calcium. John Wiley; New York: 2014.
-
- Case RM, et al. Evolution of calcium homeostasis: From birth of the first cell to an omnipresent signalling system. Cell Calcium. 2007;42:345–350. - PubMed
-
- Docampo R, de Souza W, Miranda K, Rohloff P, Moreno SNJ. Acidocalcisomes–Conserved from bacteria to man. Nat Rev Microbiol. 2005;3:251–261. - PubMed
-
- Yagisawa F, et al. Identification of novel proteins in isolated polyphosphate vacuoles in the primitive red alga Cyanidioschyzon merolae. Plant J. 2009;60:882–893. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

