Noncanonical autophagy at ER exit sites regulates procollagen turnover
- PMID: 30287488
- PMCID: PMC6205486
- DOI: 10.1073/pnas.1814552115
Noncanonical autophagy at ER exit sites regulates procollagen turnover
Abstract
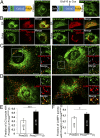
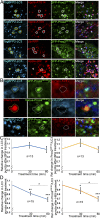
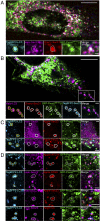
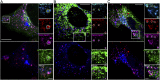
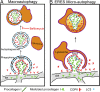
Type I collagen is the main component of bone matrix and other connective tissues. Rerouting of its procollagen precursor to a degradative pathway is crucial for osteoblast survival in pathologies involving excessive intracellular buildup of procollagen that is improperly folded and/or trafficked. What cellular mechanisms underlie this rerouting remains unclear. To study these mechanisms, we employed live-cell imaging and correlative light and electron microscopy (CLEM) to examine procollagen trafficking both in wild-type mouse osteoblasts and osteoblasts expressing a bone pathology-causing mutant procollagen. We found that although most procollagen molecules successfully trafficked through the secretory pathway in these cells, a subpopulation did not. The latter molecules appeared in numerous dispersed puncta colocalizing with COPII subunits, autophagy markers and ubiquitin machinery, with more puncta seen in mutant procollagen-expressing cells. Blocking endoplasmic reticulum exit site (ERES) formation suppressed the number of these puncta, suggesting they formed after procollagen entry into ERESs. The punctate structures containing procollagen, COPII, and autophagic markers did not move toward the Golgi but instead were relatively immobile. They appeared to be quickly engulfed by nearby lysosomes through a bafilomycin-insensitive pathway. CLEM and fluorescence recovery after photobleaching experiments suggested engulfment occurred through a noncanonical form of autophagy resembling microautophagy of ERESs. Overall, our findings reveal that a subset of procollagen molecules is directed toward lysosomal degradation through an autophagic pathway originating at ERESs, providing a mechanism to remove excess procollagen from cells.
Keywords: ERES; autophagy; lysosome; microautophagy; procollagen.
Copyright © 2018 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Noncanonical ER-Golgi trafficking and autophagy of endogenous procollagen in osteoblasts.Cell Mol Life Sci. 2021 Dec;78(24):8283-8300. doi: 10.1007/s00018-021-04017-z. Epub 2021 Nov 15. Cell Mol Life Sci. 2021. PMID: 34779895 Free PMC article.
-
Mechanisms of procollagen and HSP47 sorting during ER-to-Golgi trafficking.Matrix Biol. 2020 Nov;93:79-94. doi: 10.1016/j.matbio.2020.06.002. Epub 2020 Jun 17. Matrix Biol. 2020. PMID: 32562852 Free PMC article.
-
COPII with ALG2 and ESCRTs control lysosome-dependent microautophagy of ER exit sites.Dev Cell. 2024 Jun 3;59(11):1410-1424.e4. doi: 10.1016/j.devcel.2024.03.027. Epub 2024 Apr 8. Dev Cell. 2024. PMID: 38593803
-
Steps in the elaboration of collagen by odontoblasts and osteoblasts.Methods Cell Biol. 1981;23:167-89. doi: 10.1016/s0091-679x(08)61498-3. Methods Cell Biol. 1981. PMID: 7035802 Review. No abstract available.
-
The soluble reticulophagy receptor CALCOCO1 is also a Golgiphagy receptor.Autophagy. 2021 Aug;17(8):2051-2052. doi: 10.1080/15548627.2021.1940610. Epub 2021 Jun 24. Autophagy. 2021. PMID: 34162311 Free PMC article. Review.
Cited by
-
Collagen's enigmatic, highly conserved N-glycan has an essential proteostatic function.Proc Natl Acad Sci U S A. 2021 Mar 9;118(10):e2026608118. doi: 10.1073/pnas.2026608118. Proc Natl Acad Sci U S A. 2021. PMID: 33674390 Free PMC article.
-
The cytoplasmic tail of human mannosidase Man1b1 contributes to catalysis-independent quality control of misfolded alpha1-antitrypsin.Proc Natl Acad Sci U S A. 2020 Oct 6;117(40):24825-24836. doi: 10.1073/pnas.1919013117. Epub 2020 Sep 21. Proc Natl Acad Sci U S A. 2020. PMID: 32958677 Free PMC article.
-
Lysosomes as coordinators of cellular catabolism, metabolic signalling and organ physiology.Nat Rev Mol Cell Biol. 2024 Mar;25(3):223-245. doi: 10.1038/s41580-023-00676-x. Epub 2023 Nov 24. Nat Rev Mol Cell Biol. 2024. PMID: 38001393 Review.
-
Co-adaptor driven assembly of a CUL3 E3 ligase complex.Mol Cell. 2022 Feb 3;82(3):585-597.e11. doi: 10.1016/j.molcel.2022.01.004. Mol Cell. 2022. PMID: 35120648 Free PMC article.
-
Autophagy and the hallmarks of aging.Ageing Res Rev. 2021 Dec;72:101468. doi: 10.1016/j.arr.2021.101468. Epub 2021 Sep 24. Ageing Res Rev. 2021. PMID: 34563704 Free PMC article. Review.
References
-
- Ishikawa Y, Bächinger HP. A molecular ensemble in the rER for procollagen maturation. Biochim Biophys Acta. 2013;1833:2479–2491. - PubMed
-
- Bienkowski RS, Gotkin MG. Control of collagen deposition in mammalian lung. Proc Soc Exp Biol Med. 1995;209:118–140. - PubMed
-
- Shapiro JR, Byers PH, Glorieux FH, Sponseller PD, eds (2014) Osteogenesis Imperfecta (Elsevier, Amsterdam), pp 555.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

