The C terminus of the bacterial multidrug transporter EmrE couples drug binding to proton release
- PMID: 30287687
- PMCID: PMC6295725
- DOI: 10.1074/jbc.RA118.005430
The C terminus of the bacterial multidrug transporter EmrE couples drug binding to proton release
Abstract
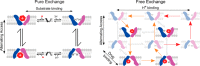
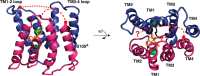
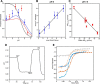
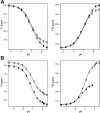
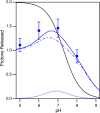
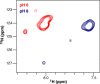
Ion-coupled transporters must regulate access of ions and substrates into and out of the binding site to actively transport substrates and minimize dissipative leak of ions. Within the single-site alternating access model, competitive substrate binding forms the foundation of ion-coupled antiport. Strict competition between substrates leads to stoichiometric antiport without slippage. However, recent NMR studies of the bacterial multidrug transporter EmrE have demonstrated that this multidrug transporter can simultaneously bind drug and proton, which will affect the transport stoichiometry and efficiency of coupled antiport. Here, we investigated the nature of substrate competition in EmrE using multiple methods to measure proton release upon the addition of saturating concentrations of drug as a function of pH. The resulting proton-release profile confirmed simultaneous binding of drug and proton, but suggested that a residue outside EmrE's Glu-14 binding site may release protons upon drug binding. Using NMR-monitored pH titrations, we trace this drug-induced deprotonation event to His-110, EmrE's C-terminal residue. Further NMR experiments disclosed that the C-terminal tail is strongly coupled to EmrE's drug-binding domain. Consideration of our results alongside those from previous studies of EmrE suggests that this conserved tail participates in secondary gating of EmrE-mediated proton/drug transport, occluding the binding pocket of fully protonated EmrE in the absence of drug to prevent dissipative proton transport.
Keywords: EmrE; antibiotics; antiporter; efflux; gating; membrane transport; multidrug transporter; nuclear magnetic resonance (NMR); proton motive forces; proton transport; structure-function.
© 2018 Thomas et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures


References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

