Role of Extracellular Matrix in Development and Cancer Progression
- PMID: 30287763
- PMCID: PMC6213383
- DOI: 10.3390/ijms19103028
Role of Extracellular Matrix in Development and Cancer Progression
Abstract
The immense diversity of extracellular matrix (ECM) proteins confers distinct biochemical and biophysical properties that influence cell phenotype. The ECM is highly dynamic as it is constantly deposited, remodelled, and degraded during development until maturity to maintain tissue homeostasis. The ECM's composition and organization are spatiotemporally regulated to control cell behaviour and differentiation, but dysregulation of ECM dynamics leads to the development of diseases such as cancer. The chemical cues presented by the ECM have been appreciated as key drivers for both development and cancer progression. However, the mechanical forces present due to the ECM have been largely ignored but recently recognized to play critical roles in disease progression and malignant cell behaviour. Here, we review the ways in which biophysical forces of the microenvironment influence biochemical regulation and cell phenotype during key stages of human development and cancer progression.
Keywords: cancer progression; extracellular matrix; fibrosis; matrix remodelling; tumour microenvironment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
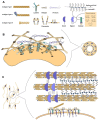
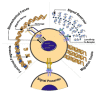

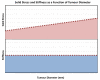
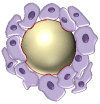

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

