The Role of Hydrogen Peroxide in Redox-Dependent Signaling: Homeostatic and Pathological Responses in Mammalian Cells
- PMID: 30287799
- PMCID: PMC6211135
- DOI: 10.3390/cells7100156
The Role of Hydrogen Peroxide in Redox-Dependent Signaling: Homeostatic and Pathological Responses in Mammalian Cells
Abstract
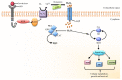
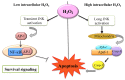
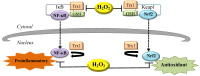
Hydrogen peroxide (H₂O₂) is an important metabolite involved in most of the redox metabolism reactions and processes of the cells. H₂O₂ is recognized as one of the main molecules in the sensing, modulation and signaling of redox metabolism, and it is acting as a second messenger together with hydrogen sulfide (H₂S) and nitric oxide (NO). These second messengers activate in turn a cascade of downstream proteins via specific oxidations leading to a metabolic response of the cell. This metabolic response can determine proliferation, survival or death of the cell depending on which downstream pathways (homeostatic, pathological, or protective) have been activated. The cells have several sources of H₂O₂ and cellular systems strictly control its concentration in different subcellular compartments. This review summarizes research on the role played by H₂O₂ in signaling pathways of eukaryotic cells and how this signaling leads to homeostatic or pathological responses.
Keywords: hydrogen peroxide; oxidative stress; redox regulation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

