Tom20 senses iron-activated ROS signaling to promote melanoma cell pyroptosis
- PMID: 30287942
- PMCID: PMC6274649
- DOI: 10.1038/s41422-018-0090-y
Tom20 senses iron-activated ROS signaling to promote melanoma cell pyroptosis
Abstract
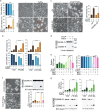
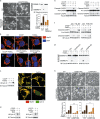
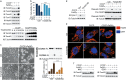
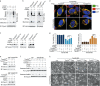
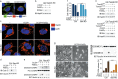
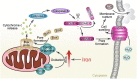
Iron has been shown to trigger oxidative stress by elevating reactive oxygen species (ROS) and to participate in different modes of cell death, such as ferroptosis, apoptosis and necroptosis. However, whether iron-elevated ROS is also linked to pyroptosis has not been reported. Here, we demonstrate that iron-activated ROS can induce pyroptosis via a Tom20-Bax-caspase-GSDME pathway. In melanoma cells, iron enhanced ROS signaling initiated by CCCP, causing the oxidation and oligomerization of the mitochondrial outer membrane protein Tom20. Bax is recruited to mitochondria by oxidized Tom20, which facilitates cytochrome c release to cytosol to activate caspase-3, eventually triggering pyroptotic death by inducing GSDME cleavage. Therefore, ROS acts as a causative factor and Tom20 senses ROS signaling for iron-driven pyroptotic death of melanoma cells. Since iron activates ROS for GSDME-dependent pyroptosis induction and melanoma cells specifically express a high level of GSDME, iron may be a potential candidate for melanoma therapy. Based on the functional mechanism of iron shown above, we further demonstrate that iron supplementation at a dosage used in iron-deficient patients is sufficient to maximize the anti-tumor effect of clinical ROS-inducing drugs to inhibit xenograft tumor growth and metastasis of melanoma cells through GSDME-dependent pyroptosis. Moreover, no obvious side effects are observed in the normal tissues and organs of mice during the combined treatment of clinical drugs and iron. This study not only identifies iron as a sensitizer amplifying ROS signaling to drive pyroptosis, but also implicates a novel iron-based intervention strategy for melanoma therapy.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

