Enantioselective counter-anions in photoredox catalysis: the asymmetric cation radical Diels-Alder reaction
- PMID: 30287974
- PMCID: PMC6166882
- DOI: 10.1016/j.tet.2018.03.052
Enantioselective counter-anions in photoredox catalysis: the asymmetric cation radical Diels-Alder reaction
Abstract
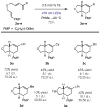
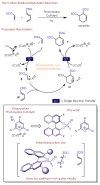
Control of absolute stereochemistry in radical and ion radical transformations is a major challenge in synthetic chemistry. Herein, we report the design of a photoredox catalyst system comprised of an oxidizing pyrilium salt bearing a chiral N-triflyl phosphoramide anion. This class of chiral organic photoredox catalysts is able to catalyze the formation of cation radical-mediated Diels-Alder transformations in up to 75:25 e.r. in both intramolecular and intermolecular examples.
Keywords: catalysis; chiral anion; cycloaddition; enantioselective; photoredox.
Figures
References
-
-
For recent reviews on photoredox catalysis see: Romero NA, Nicewicz DA. Chem Rev. 2016;116:10075–10166.Prier CK, Rankic DA, MacMillan DWC. Chem Rev. 2013;113:5322–5363.Shaw MH, Twilton J, MacMillan DWC. J Org Chem. 2016;81:6898–6926.
-
-
- Nicewicz DA, MacMillan DWC. Science. 2008;322:77–80. - PMC - PubMed
- Nagib DA, Scott ME, MacMillan DWC. J Am Chem Soc. 2009;131:10875–10877. - PMC - PubMed
- Shih HW, Vander Wal MN, Grange RL, MacMillan DWC. J Am Chem Soc. 2010;132:13600–13603. - PMC - PubMed
- Welin ER, Warkentin AA, Conrad JC, MacMillan DWC. Angew Chem Int Ed. 2015;54:9668–9672. - PMC - PubMed
-
- Rono LJ, Yayla HG, Wang DY, Armstrong MF, Knowles RR. J Am Chem Soc. 2013;135:17735–17738. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources