CPEB4 promotes growth and metastasis of gastric cancer cells via ZEB1-mediated epithelial- mesenchymal transition
- PMID: 30288051
- PMCID: PMC6160272
- DOI: 10.2147/OTT.S175428
CPEB4 promotes growth and metastasis of gastric cancer cells via ZEB1-mediated epithelial- mesenchymal transition
Abstract
Background: Cytoplasmic polyadenylation element-binding protein 4 (CPEB4) has previously been reported to be associated with biological malignancy in various cancers. However, its function in tumor growth and metastasis in gastric cancer (GC) remains obscure. Here, we explored the functional and molecular mechanisms by which CPEB4 influences GC.
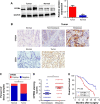
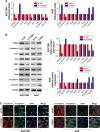
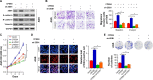
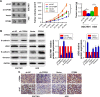
Materials and methods: The expression of CPEB4 was assessed using Western blot and immunohistochemistry in GC specimens. The roles of CPEB4 in GC cell proliferation, migration, and invasion were investigated by cell-counting kit-8 (CCK-8), colony formation, and EdU assay; wound-healing assay; and transwell assay, respectively. Quantitative real-time PCR (qRT-PCR), Western blot, and immunofluorescence staining were performed to detect the expressions of CPEB4 and epithelial-mesenchymal transition (EMT)-related markers. The function of CPEB4 on GC cell growth and metastasis was also determined in vivo through establishing subcutaneous xenograft tumor and lung metastatic mice model.
Results: The results revealed that the expression of CPEB4 was increased in GC tissues compared with matched normal tissues. High expression level of CPEB4 was significantly associated with clinical metastasis and unfavorable prognosis in patients with GC. Furthermore, CPEB4 silencing remarkably inhibited GC cells' proliferation, invasion, and metastasis in vitro and in vivo. Conversely, CPEB4 overexpression achieved the opposite effects. Mechanically, we proved that ZEB1-mediated EMT might be involved in CPEB4-facilitated GC cells' proliferation, invasion, and metastasis.
Conclusion: Our findings implied that CPEB4 expression predicted a worse prognosis in patients with GC. Besides, CPEB4 contributed to GC cells' proliferation, migration, and invasion via ZEB1-mediated EMT.
Keywords: CPEB4; epithelial-mesenchymal transition; gastric cancer.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures


Similar articles
-
SOX5 promotes cell invasion and metastasis via activation of Twist-mediated epithelial-mesenchymal transition in gastric cancer.Onco Targets Ther. 2019 Apr 3;12:2465-2476. doi: 10.2147/OTT.S197087. eCollection 2019. Onco Targets Ther. 2019. PMID: 31040690 Free PMC article.
-
TRIM37 promotes cell invasion and metastasis by regulating SIP1-mediated epithelial-mesenchymal transition in gastric cancer.Onco Targets Ther. 2018 Dec 10;11:8803-8813. doi: 10.2147/OTT.S178446. eCollection 2018. Onco Targets Ther. 2018. PMID: 30573971 Free PMC article.
-
A-kinase-interacting protein 1 facilitates growth and metastasis of gastric cancer cells via Slug-induced epithelial-mesenchymal transition.J Cell Mol Med. 2019 Jun;23(6):4434-4442. doi: 10.1111/jcmm.14339. Epub 2019 Apr 24. J Cell Mol Med. 2019. PMID: 31020809 Free PMC article.
-
Implications of the Receptor Tyrosine Kinase Axl in Gastric Cancer Progression.Onco Targets Ther. 2020 Jun 22;13:5901-5911. doi: 10.2147/OTT.S257606. eCollection 2020. Onco Targets Ther. 2020. Retraction in: Onco Targets Ther. 2024 Aug 30;17:727-728. doi: 10.2147/OTT.S493699. PMID: 32606800 Free PMC article. Retracted.
-
Interleukin-34 promotes the proliferation and epithelial-mesenchymal transition of gastric cancer cells.World J Gastrointest Oncol. 2022 Oct 15;14(10):1968-1980. doi: 10.4251/wjgo.v14.i10.1968. World J Gastrointest Oncol. 2022. PMID: 36310707 Free PMC article.
Cited by
-
MSC-AS1 knockdown inhibits cell growth and temozolomide resistance by regulating miR-373-3p/CPEB4 axis in glioma through PI3K/Akt pathway.Mol Cell Biochem. 2021 Feb;476(2):699-713. doi: 10.1007/s11010-020-03937-x. Epub 2020 Oct 26. Mol Cell Biochem. 2021. PMID: 33106913 Free PMC article.
-
Antitumor T-cell function requires CPEB4-mediated adaptation to chronic endoplasmic reticulum stress.EMBO J. 2023 May 2;42(9):e111494. doi: 10.15252/embj.2022111494. Epub 2023 Mar 15. EMBO J. 2023. PMID: 36919984 Free PMC article.
-
CircESRP1 enhances metastasis and epithelial-mesenchymal transition in endometrial cancer via the miR-874-3p/CPEB4 axis.J Transl Med. 2022 Mar 22;20(1):139. doi: 10.1186/s12967-022-03334-6. J Transl Med. 2022. PMID: 35317822 Free PMC article.
-
Cytoplasmic regulation of the poly(A) tail length as a potential therapeutic target.RNA. 2025 Feb 19;31(3):402-415. doi: 10.1261/rna.080333.124. RNA. 2025. PMID: 39805658 Free PMC article. Review.
-
Circular RNA Circ_0003221 Promotes Cervical Cancer Progression by Regulating miR-758-3p/CPEB4 Axis.Cancer Manag Res. 2021 Jul 5;13:5337-5350. doi: 10.2147/CMAR.S311242. eCollection 2021. Cancer Manag Res. 2021. PMID: 34262342 Free PMC article.
References
-
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–E386. - PubMed
-
- Fock KM. Review article: the epidemiology and prevention of gastric cancer. Aliment Pharmacol Ther. 2014;40(3):250–260. - PubMed
-
- Catalano V, Labianca R, Beretta GD, et al. Gastric cancer. Crit Rev Oncol Hematol. 2009;71(2):127–164. - PubMed
-
- Davis FM, Stewart TA, Thompson EW, Monteith GR. Targeting EMT in cancer: opportunities for pharmacological intervention. Trends Pharmacol Sci. 2014;35(9):479–488. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

