Spotlight on the NLRP3 inflammasome pathway
- PMID: 30288079
- PMCID: PMC6161739
- DOI: 10.2147/JIR.S141220
Spotlight on the NLRP3 inflammasome pathway
Abstract
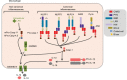
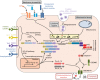
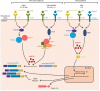
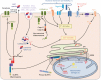
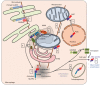
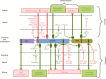
Inflammation is triggered by a repertoire of receptors detecting infections and damages. Some of these receptors directly bind microbial ligands, while others recognize endogenous molecules exposed under stress conditions, including infections. Most of these receptors can be engaged by a relatively limited number of stimuli. Differently, NLRP3 acts as a broad sensor of cell homeostasis rupture and can be activated downstream of a plethora of stimuli. NLRP3 then assembles a multiprotein platform resulting in caspase-1 activation, which controls, by direct cleavage, the maturation of cytosolic pro-cytokines including pro-interleukin-1β. In addition, caspase-1 processes cytosolic gasdermin-D and unleashes its pore-forming N-terminal domain, leading to the release of mature cytosolic cytokines and alarmins, as well as pyroptotic cell lysis. Accumulating evidences of the aggravating role of NLRP3-mediated inflammation in various highly prevalent human conditions, including diabetes, neurodegenerative and cardiovascular diseases, raises a huge clinical interest. Nevertheless, the molecular mechanism governing NLRP3 activation remains insufficiently understood. In line with the detrimental consequences of NLRP3 activation illustrated by the aforementioned pathologies, this process is tightly regulated. In this review, we address the current understanding of the control of NLRP3 activity which can be divided into two coordinated processes referred to as priming and activation. In particular, we detail the emerging role of NLRP3 post-translational modifications critical in inflammasome assembly regulation.
Keywords: NLRP3; inflammasome; inflammation; post-translational modifications.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures
References
-
- Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454(7203):428–435. - PubMed
-
- Medzhitov R, Janeway CA. Innate immunity: the virtues of a nonclonal system of recognition. Cell. 1997;91(3):295–298. - PubMed
-
- Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. - PubMed
-
- Hu Z, Zhou Q, Zhang C, et al. Structural and biochemical basis for induced self-propagation of NLRC4. Science. 2015;350(6259):399–404. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

