Targeting EGFR and uPAR on human rhabdomyosarcoma, osteosarcoma, and ovarian adenocarcinoma with a bispecific ligand-directed toxin
- PMID: 30288129
- PMCID: PMC6163021
- DOI: 10.2147/CPAA.S160262
Targeting EGFR and uPAR on human rhabdomyosarcoma, osteosarcoma, and ovarian adenocarcinoma with a bispecific ligand-directed toxin
Abstract
Purpose: Human sarcomas are rare and difficult to treat cancerous tumors typically arising from soft tissue or bone. Conversely, carcinomas are the most common cancer subtype in humans and the primary cause of mortality across all cancer patients. While conventional therapeutic modalities can prolong disease-free intervals and survival in some cases, treatment of refractory or recurrent solid tumors is challenging, and tumor-related mortality remains unacceptably high. The identification of overexpressed cell surface receptors on sarcoma and carcinoma cells has provided a valuable tool to develop targeted toxins as an alternative anticancer strategy. Recent investigation of recombinant protein-linked toxins that specifically target these cancer receptors has led to the development of highly specific, cytotoxic, and deimmunized drugs that can kill cancer cells.
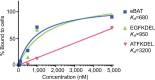
Methods: This study investigated a recombinant protein called epidermal growth factor bispecific angiotoxin (eBAT), which is designed to target the epidermal growth factor receptor (EGFR) on cancer cells and the urokinase plasminogen activator receptor (uPAR) on cancer cells and associated tumor vasculature. Both receptors are expressed by a variety of human sarcomas and carcinomas. Flow cytometry techniques were used to determine binding affinity of eBAT to cancer cells, and proliferation assays were performed to calculate tumor killing ability based on half-maximal inhibitory concentrations.
Results: eBAT demonstrated cytotoxicity against a variety of sarcoma and carcinoma cells that overexpress EGFR and uPAR in vitro and showed greater cell killing ability and binding affinity to cancer cells compared with its monospecific counterparts.
Conclusion: The results of our study are promising, and further studies will be necessary to confirm the applicability of eBAT as a supplementary therapy for a variety of sarcomas, carcinomas, and possibly other refractory malignancies that express EGFR and uPAR.
Keywords: EGFR; carcinoma; eBAT; sarcoma; uPAR.
Conflict of interest statement
Disclosure Dr Vallera is a member of the Oxis Biotech Scientific Advisory Board and holds equity in the company. This relation ship has been reviewed and managed by the University of Minnesota in accordance with its conflict of interest policies. A Borgatti and D Vallera have ownership interest (including patents) in a patent entitled “Reduction of EGFR therapeutic toxicity” filed by the University of Minnesota Office of Technology Commercialization. The other authors report no conflicts of interest in this work.
Figures



References
-
- World Health Organization . Classification of tumours. In: Fletcher CDM, Unni KK, Mertens F, editors. Pathology and Genetics of Tumors of Soft Tissue. Lyon, France: IARC Press; 2002. pp. 12–224.
-
- American Cancer Society . Cancer Facts & Figures. Atlanta, Ga: American Cancer Society; 2018. 2018.
-
- Rosenberg SA, Tepper J, Glatstein E, et al. The treatment of soft-tissue sarcomas of the extremities: prospective randomized evaluations of (1) limb-sparing surgery plus radiation therapy compared with amputation and (2) the role of adjuvant chemotherapy. Ann Surg. 1982;196(3):305–315. - PMC - PubMed
-
- Survival rates for ovarian cancer, by stage 2018. [Accessed April 17, 2018]. Available from: https://www.cancer.org/cancer/ovarian-cancer/detection-diagnosis-staging....
-
- Dorsam RT, Gutkind JS. G-protein-coupled receptors and cancer. Nat Rev Cancer. 2007;7(2):79–94. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

