Endocannabinoids and related N-acylethanolamines: biological activities and metabolism
- PMID: 30288203
- PMCID: PMC6166290
- DOI: 10.1186/s41232-018-0086-5
Endocannabinoids and related N-acylethanolamines: biological activities and metabolism
Abstract
The plant Cannabis sativa contains cannabinoids represented by Δ9-tetrahydrocannabinol, which exert psychoactivity and immunomodulation through cannabinoid CB1 and CB2 receptors, respectively, in animal tissues. Arachidonoylethanolamide (also referred to as anandamide) and 2-arachidonoylglycerol (2-AG) are well known as two major endogenous agonists of these receptors (termed "endocannabinoids") and show various cannabimimetic bioactivities. However, only 2-AG is a full agonist for CB1 and CB2 and mediates retrograde signals at the synapse, strongly suggesting that 2-AG is physiologically more important than anandamide. The metabolic pathways of these two endocannabinoids are completely different. 2-AG is mostly produced from inositol phospholipids via diacylglycerol by phospholipase C and diacylglycerol lipase and then degraded by monoacylglycerol lipase. On the other hand, anandamide is concomitantly produced with larger amounts of other N-acylethanolamines via N-acyl-phosphatidylethanolamines (NAPEs). Although this pathway consists of calcium-dependent N-acyltransferase and NAPE-hydrolyzing phospholipase D, recent studies revealed the involvement of several new enzymes. Quantitatively major N-acylethanolamines include palmitoylethanolamide and oleoylethanolamide, which do not bind to cannabinoid receptors but exert anti-inflammatory, analgesic, and anorexic effects through receptors such as peroxisome proliferator-activated receptor α. The biosynthesis of these non-endocannabinoid N-acylethanolamines rather than anandamide may be the primary significance of this pathway. Here, we provide an overview of the biological activities and metabolisms of endocannabinoids (2-AG and anandamide) and non-endocannabinoid N-acylethanolamines.
Keywords: 2-Arachidonoylglycerol; Anandamide; Endocannabinoid; Lipid mediator; Metabolism; N-Acylethanolamine; Phospholipase; Phospholipid.
Conflict of interest statement
Not applicable.Not applicable.The authors declare that they have no competing interests.Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
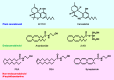


References
-
- Gaoni Y, Mechoulam R. Isolation, structure and partial synthesis of an active constituent of hashish. J Am Chem Soc. 1964;86:1646–1647. doi: 10.1021/ja01062a046. - DOI
-
- Devane WA, Dysarz FA, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol. 1988;34:605–613. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

