β-lactam/β-lactamase inhibitor combinations: an update
- PMID: 30288219
- PMCID: PMC6151480
- DOI: 10.1039/c8md00342d
β-lactam/β-lactamase inhibitor combinations: an update
Abstract
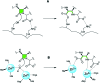
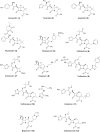
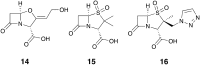
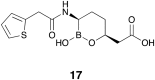
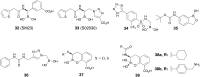
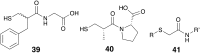
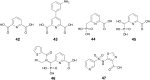
Antibiotic resistance caused by β-lactamase production continues to present a growing challenge to the efficacy of β-lactams and their role as the most important class of clinically used antibiotics. In response to this threat however, only a handful of β-lactamase inhibitors have been introduced to the market over the past thirty years. The first-generation β-lactamase inhibitors (clavulanic acid, sulbactam and tazobactam) are all β-lactam derivatives and work primarily by inactivating class A and some class C serine β-lactamases. The newer generations of β-lactamase inhibitors including avibactam and vaborbactam are based on non-β-lactam structures and their spectrum of inhibition is extended to KPC as an important class A carbapenemase. Despite these advances several class D and virtually all important class B β-lactamases are resistant to existing inhibitors. The present review provides an overview of recent FDA-approved β-lactam/β-lactamase inhibitor combinations as well as an update on research efforts aimed at the discovery and development of novel β-lactamase inhibitors.
Figures
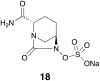
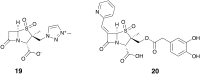
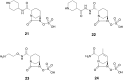
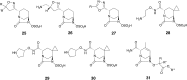



References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

