Enantioselective aza-Friedel-Crafts reaction of furan with α-ketimino esters induced by a conjugated double hydrogen bond network of chiral bis(phosphoric acid) catalysts
- PMID: 30288231
- PMCID: PMC6143995
- DOI: 10.1039/c8sc02290a
Enantioselective aza-Friedel-Crafts reaction of furan with α-ketimino esters induced by a conjugated double hydrogen bond network of chiral bis(phosphoric acid) catalysts
Abstract
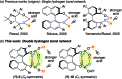
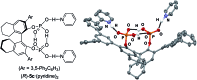
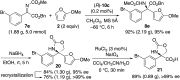
Chiral C 2- and C 1-symmetric BINOL-derived bis(phosphoric acid) catalysts, which have OP([double bond, length as m-dash]O)(OH)2/OP([double bond, length as m-dash]O)(OH)(OR) moieties at the 2,2'-positions, were developed and used for the enantioselective aza-Friedel-Crafts reaction of 2-methoxyfuran with α-ketimino esters for the first time. The intramolecular conjugated double hydrogen bond network is a key to increasing the Brønsted acidity and preventing deactivation of the catalysts. Highly functionalized α-amino acid derivatives with a chiral quaternary carbon center could be transformed into versatile optically active N- and O-heterocycles and an α-aryl-substituted serine.
This journal is © The Royal Society of Chemistry 2018.
Figures




References
-
-
For reviews on chiral Brønsted acids.
- Akiyama T. Chem. Rev. 2007;107:5744. - PubMed
- Terada M. Synthesis. 2010;2010:1929.
- Kampen D., Reisinger C. M., List B. Top. Curr. Chem. 2010;291:395. - PubMed
- Parmar D., Sugiono E., Raja S., Rueping M. Chem. Rev. 2014;114:9047. - PubMed
- Akiyama T., Mori K. Chem. Rev. 2015;115:9277. - PubMed
- Parmar D., Sugiono E., Raja S., Rueping M. Chem. Rev. 2017;117:10608. - PubMed
- Merad J., Lalli C., Bernadat G., Maury J., Masson G. Chem.–Eur. J. 2018;24:3925. - PubMed
-
-
- Huang Y., Unni A. K., Thadani A. N., Rawal V. H. Nature. 2003;424:146. - PubMed
- McDougal N. T., Schaus S. E. J. Am. Chem. Soc. 2003;125:12094. - PubMed
- Thadani A. N., Stankovic A. R., Rawal V. H. Proc. Natl. Acad. Sci. U. S. A. 2004;101:5846. - PMC - PubMed
- Unni A. K., Takenaka N., Yamamoto H., Rawal V. H. J. Am. Chem. Soc. 2005;127:1336. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

