Beneficial coinfection can promote within-host viral diversity
- PMID: 30288300
- PMCID: PMC6166523
- DOI: 10.1093/ve/vey028
Beneficial coinfection can promote within-host viral diversity
Abstract
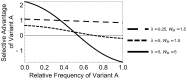
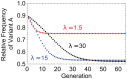
In many viral infections, a large number of different genetic variants can coexist within a host, leading to more virulent infections that are better able to evolve antiviral resistance and adapt to new hosts. But how is this diversity maintained? Why do faster-growing variants not outcompete slower-growing variants, and erode this diversity? One hypothesis is if there are mutually beneficial interactions between variants, with host cells infected by multiple different viral genomes producing more, or more effective, virions. We modelled this hypothesis with both mathematical models and simulations, and found that moderate levels of beneficial coinfection can maintain high levels of coexistence, even when coinfection is relatively rare, and when there are significant fitness differences between competing variants. Rare variants are more likely to be coinfecting with a different variant, and hence beneficial coinfection increases the relative fitness of rare variants through negative frequency dependence, and maintains diversity. We further find that coexisting variants sometimes reach unequal frequencies, depending on the extent to which different variants benefit from coinfection, and the ratio of variants which leads to the most productive infected cells. These factors could help drive the evolution of defective interfering particles, and help to explain why the different segments of multipartite viruses persist at different equilibrium frequencies.
Keywords: coinfection; diversity; evolution; frequency dependence; multipartite; phenotype mixing.
Figures
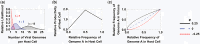


Similar articles
-
Influenza A Virus Coinfection through Transmission Can Support High Levels of Reassortment.J Virol. 2015 Aug;89(16):8453-61. doi: 10.1128/JVI.01162-15. Epub 2015 Jun 3. J Virol. 2015. PMID: 26041285 Free PMC article.
-
Cheating leads to the evolution of multipartite viruses.PLoS Biol. 2023 Apr 24;21(4):e3002092. doi: 10.1371/journal.pbio.3002092. eCollection 2023 Apr. PLoS Biol. 2023. PMID: 37093882 Free PMC article.
-
Fitness Estimation for Viral Variants in the Context of Cellular Coinfection.Viruses. 2021 Jun 23;13(7):1216. doi: 10.3390/v13071216. Viruses. 2021. PMID: 34201862 Free PMC article.
-
From viral democratic genomes to viral wild bunch of quasispecies.J Med Virol. 2023 Nov;95(11):e29209. doi: 10.1002/jmv.29209. J Med Virol. 2023. PMID: 37937701 Review.
-
Strength in numbers: Mechanisms of viral co-infection.Virus Res. 2019 May;265:43-46. doi: 10.1016/j.virusres.2019.03.003. Epub 2019 Mar 2. Virus Res. 2019. PMID: 30836113 Free PMC article. Review.
Cited by
-
Uncovering Virus-Virus Interactions by Unifying Approaches and Harnessing High-Throughput Tools.mSystems. 2019 Jun 4;4(3):e00121-19. doi: 10.1128/mSystems.00121-19. mSystems. 2019. PMID: 31164405 Free PMC article.
-
Diverse phage communities are maintained stably on a clonal bacterial host.Science. 2024 Dec 13;386(6727):1294-1300. doi: 10.1126/science.adk1183. Epub 2024 Dec 12. Science. 2024. PMID: 39666794 Free PMC article.
-
Vector-virus interaction affects viral loads and co-occurrence.BMC Biol. 2022 Dec 17;20(1):284. doi: 10.1186/s12915-022-01463-4. BMC Biol. 2022. PMID: 36527054 Free PMC article.
-
Collective Viral Spread Mediated by Virion Aggregates Promotes the Evolution of Defective Interfering Particles.mBio. 2020 Jan 7;11(1):e02156-19. doi: 10.1128/mBio.02156-19. mBio. 2020. PMID: 31911487 Free PMC article.
-
Membrane-Associated Enteroviruses Undergo Intercellular Transmission as Pools of Sibling Viral Genomes.Cell Rep. 2019 Oct 15;29(3):714-723.e4. doi: 10.1016/j.celrep.2019.09.014. Cell Rep. 2019. PMID: 31618638 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

