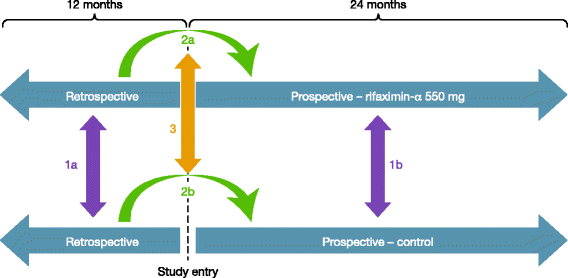
Design of the Prospective Real-world Outcomes Study of hepatic encephalopathy Patients' Experience on Rifaximin-α (PROSPER): an observational study among 550 patients
- PMID: 30288327
- PMCID: PMC5918574
- DOI: 10.1186/s41124-017-0029-9
Design of the Prospective Real-world Outcomes Study of hepatic encephalopathy Patients' Experience on Rifaximin-α (PROSPER): an observational study among 550 patients
Abstract
Background: Hepatic encephalopathy (HE) is one of the most important severe complications of liver cirrhosis. Thought to be caused by elevated blood levels of gut-derived neurotoxins (particularly ammonia) entering the brain, HE manifests as a wide range of neurological or psychiatric abnormalities, which increase the risk of mortality, result in substantial morbidity and negatively affect the quality of life (QoL) of both patients and their caregivers. HE is also associated with a substantial economic burden. Rifaximin-α 550 mg is a locally acting oral antibiotic that reduces the effects of ammonia-producing intestinal flora, and which is used to help reduce the recurrence of overt HE. The efficacy of rifaximin-α 550 mg was established in a randomised controlled trial and long-term extension study. However, 'real-world' evidence is also required to assess how this efficacy may translate into effectiveness in clinical practice, including the potential impact of treatment on healthcare resource utilisation.
Methods: The Prospective Real-world Outcomes Study of HE Patients' Experience on Rifaximin-α 550 mg (PROSPER) is a multinational, multicentre, observational study that will be conducted under real-world clinical practice conditions. Comprising a retrospective phase (up to 12 months) and a prospective phase (up to 24 months), and employing a robust statistical methodology, PROSPER has been specifically designed to minimise the bias associated with observational studies. The primary endpoint will be the effect of rifaximin-α 550 mg treatment on HE- and liver-related hospitalisation rate and duration of hospitalisation. Secondary endpoints will include comprehensive assessments of the impact of treatment on the QoL and workplace productivity of patients and caregivers, a global assessment of treatment effectiveness and safety/tolerability. Approximately 550 patients will be enrolled.
Conclusions: PROSPER will provide valuable real-world information on the effectiveness of rifaximin-α 550 mg in reducing the recurrence of HE, and its impact on the QoL and work productivity of patients and their caregivers. By providing data on both the direct costs (e.g., hospitalisation rate, duration of hospitalisation) and indirect costs (such as work productivity) of HE, PROSPER should help confirm whether rifaximin-α 550 mg treatment represents a good use of economic resources.
Trial registration: ClinicalTrials.gov identifier NCT02488993.
Keywords: Cirrhosis; Health economics; Hepatic encephalopathy; Hospitalisation; Liver disease; Patient-reported outcomes; Quality of life; Real-world; Rifaximin-α 550 mg; Work productivity.
Conflict of interest statement
The principal country for patient recruitment will be Denmark, where no ethical approval is required for observational studies. In other countries, where appropriate, the Principal Investigator or designee at each study site will obtain independent ethics committee review and approval of the study protocol, informed consent forms and any other relevant documents before any study-related activities involving patients are performed. The study will comply with all applicable laws, regulations and guidance regarding patient protection, including patient privacy.Not applicable.AK: Norgine advisory board, speaker and investigator. MS: Lecture and travel fees from AbbVie, Gilead, Falk and Norgine; in addition, advisory board honoraria from AbbVie and Norgine. HS, JP and JW: Employees of Norgine Ltd. SIS: Advisory board membership/speaker fees from Norgine, Gilead Sciences, AbbVie, Bristol Myers Squibb, MSD, Bayer Healthcare and Astellas. MH: Speaker fees and travel expenses from Norgine, Astellas, Janssen and AbbVie.Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- American Association for the Study of Liver Diseases; European Association for the Study of the Liver. Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the European Association for the Study of the Liver and the American Association for the Study of Liver Diseases. J Hepatol. 2014;61:642–59. - PubMed
-
- Cordoba J, Ventura-Cots M, Simón-Talero M, Amorós À, Pavesi M, Vilstrup H, Angeli P, Domenicali M, Ginés P, Bernardi M, Arroyo V, CANONIC Study Investigators of EASL-CLIF consortium. Characteristics, risk factors, and mortality of cirrhotic patients hospitalized for hepatic encephalopathy with and without acute-on-chronic liver failure (ACLF). J Hepatol. 2014;60:275–81. - PubMed
-
- Bajaj JS, Wade JB, Gibson DP, Heuman DM, Thacker LR, Sterling RK, Stravitz RT, Luketic V, Fuchs M, White MB, Bell DE, Gilles H, Morton K, Noble N, Puri P, Sanyal AJ. The multi-dimensional burden of cirrhosis and hepatic encephalopathy on patients and caregivers. Am J Gastroenterol. 2011;106:1646–1653. doi: 10.1038/ajg.2011.157. - DOI - PMC - PubMed
-
- Aspinall R, Radwan A, Shaya G, Sodatonou H, Cipelli R, Hudson M. The impact of rifaximin-alpha on NHS hospital resource use in UK patients with hepatic encephalopathy: a retrospective observational study (IMPRESS). J Hepatol. 2016;64(Suppl 2):S283 (abstract THU-376).
-
- Bajaj JS, Reddy KR, Tandon P, Wong F, Kamath PS, Garcia-Tsao G, Maliakkal B, Biggins SW, Thuluvath PJ, Fallon MB, Subramanian RM, Vargas H, Thacker LR, O'Leary JG. North American Consortium for the Study of End-Stage Liver Disease. The 3-month readmission rate remains unacceptably high in a large North American cohort of cirrhotic patients. Hepatology. 2016;64:200–8. - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical