Treatment-naïve HPV+ head and neck cancers display a T-cell-inflamed phenotype distinct from their HPV- counterparts that has implications for immunotherapy
- PMID: 30288365
- PMCID: PMC6169583
- DOI: 10.1080/2162402X.2018.1498439
Treatment-naïve HPV+ head and neck cancers display a T-cell-inflamed phenotype distinct from their HPV- counterparts that has implications for immunotherapy
Abstract
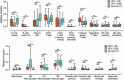
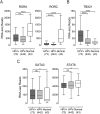
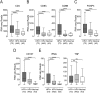
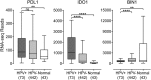
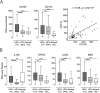
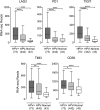
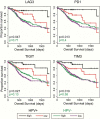
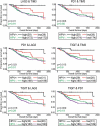
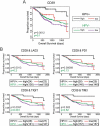
Cancers progress when the immune system fails to identify and eliminate malignant cells. Recognition of this, combined with advances in tumor immunology, has allowed development of therapies that induce effective anti-tumor immune responses. For incompletely-understood reasons, effective responses to immunotherapy occur in some patients and not others. Head and neck squamous cell carcinomas (HNSCC) are a common cancer type that can be divided into two subsets based on human papillomavirus (HPV) status. HPV status is a strong predictor of positive clinical outcome. Expression of exogenous viral antigens by HPV+, but not HPV-, HNSCC allows direct comparison of the immune status (immune cell presence and characteristics) between these two otherwise anatomically-similar tumors. Using TCGA data, we compared the immune landscape between HPV+ and HPV- treatment-naïve HNSCC. As compared to HPV- samples, HPV+ HNSCC exhibited a strong Th1 response characterized by increased infiltration with multiple types of immune cells and expression of their effector molecules. HPV+ HNSCC also expressed higher levels of CD39 and multiple T-cell exhaustion markers including LAG3, PD1, TIGIT, and TIM3 compared to HPV- HNSCC. Importantly, patients with higher expression of these exhaustion markers-indicative of a T-cell-inflamed tumor-correlated with markedly improved survival in HPV+, but not HPV-, HNSCC. Thus, profound differences exist between the immune landscape of HPV+ and HPV- HNSCC. These results suggest that immune checkpoint inhibitor therapy is a promising treatment strategy for HPV+ HNSCC, and that expression of immune checkpoint molecules could serve as a predictive biomarker of patient outcome in HPV+ HNSCC.
Keywords: head and neck cancer; human papillomavirus; immune checkpoint markers; survival; tumour infiltrating lymphocytes.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
